Cross-Talk between Carbon Metabolism and the DNA Damage Response in S. cerevisiae
- PMID: 26344768
- PMCID: PMC4581987
- DOI: 10.1016/j.celrep.2015.08.025
Cross-Talk between Carbon Metabolism and the DNA Damage Response in S. cerevisiae
Abstract
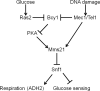
Yeast cells with DNA damage avoid respiration, presumably because products of oxidative metabolism can be harmful to DNA. We show that DNA damage inhibits the activity of the Snf1 (AMP-activated) protein kinase (AMPK), which activates expression of genes required for respiration. Glucose and DNA damage upregulate SUMOylation of Snf1, catalyzed by the SUMO E3 ligase Mms21, which inhibits SNF1 activity. The DNA damage checkpoint kinases Mec1/ATR and Tel1/ATM, as well as the nutrient-sensing protein kinase A (PKA), regulate Mms21 activity toward Snf1. Mec1 and Tel1 are required for two SNF1-regulated processes-glucose sensing and ADH2 gene expression-even without exogenous genotoxic stress. Our results imply that inhibition of Snf1 by SUMOylation is a mechanism by which cells lower their respiration in response to DNA damage. This raises the possibility that activation of DNA damage checkpoint mechanisms could contribute to aerobic fermentation (Warburg effect), a hallmark of cancer cells.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
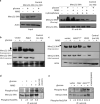
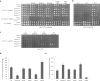
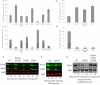

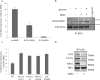


Similar articles
-
SUMOylation regulates the SNF1 protein kinase.Proc Natl Acad Sci U S A. 2013 Oct 22;110(43):17432-7. doi: 10.1073/pnas.1304839110. Epub 2013 Oct 9. Proc Natl Acad Sci U S A. 2013. PMID: 24108357 Free PMC article.
-
Mec1-dependent phosphorylation of Mms21 modulates its SUMO ligase activity.DNA Repair (Amst). 2015 Apr;28:83-92. doi: 10.1016/j.dnarep.2015.01.006. Epub 2015 Jan 30. DNA Repair (Amst). 2015. PMID: 25659338
-
Snf1 Phosphorylates Adenylate Cyclase and Negatively Regulates Protein Kinase A-dependent Transcription in Saccharomyces cerevisiae.J Biol Chem. 2015 Oct 9;290(41):24715-26. doi: 10.1074/jbc.M115.658005. Epub 2015 Aug 26. J Biol Chem. 2015. PMID: 26309257 Free PMC article.
-
Interplays between ATM/Tel1 and ATR/Mec1 in sensing and signaling DNA double-strand breaks.DNA Repair (Amst). 2013 Oct;12(10):791-9. doi: 10.1016/j.dnarep.2013.07.009. Epub 2013 Aug 13. DNA Repair (Amst). 2013. PMID: 23953933 Review.
-
Cell-cycle-specific activators of the Mec1/ATR checkpoint kinase.Biochem Soc Trans. 2011 Apr;39(2):600-5. doi: 10.1042/BST0390600. Biochem Soc Trans. 2011. PMID: 21428947 Review.
Cited by
-
Spontaneous mutations in CYC8 and MIG1 suppress the short chronological lifespan of budding yeast lacking SNF1/AMPK.Microb Cell. 2018 Feb 19;5(5):233-248. doi: 10.15698/mic2018.05.630. Microb Cell. 2018. PMID: 29796388 Free PMC article.
-
Saccharomyces cerevisiae as a Model System for Eukaryotic Cell Biology, from Cell Cycle Control to DNA Damage Response.Int J Mol Sci. 2022 Oct 1;23(19):11665. doi: 10.3390/ijms231911665. Int J Mol Sci. 2022. PMID: 36232965 Free PMC article. Review.
-
Antagonism between salicylate and the cAMP signal controls yeast cell survival and growth recovery from quiescence.Microb Cell. 2018 Mar 26;5(7):344-356. doi: 10.15698/mic2018.07.640. Microb Cell. 2018. PMID: 29992130 Free PMC article.
-
Low RNA Polymerase III activity results in up regulation of HXT2 glucose transporter independently of glucose signaling and despite changing environment.PLoS One. 2017 Sep 29;12(9):e0185516. doi: 10.1371/journal.pone.0185516. eCollection 2017. PLoS One. 2017. PMID: 28961268 Free PMC article.
-
Regulation of yeast Snf1 (AMPK) by a polyhistidine containing pH sensing module.iScience. 2022 Sep 6;25(10):105083. doi: 10.1016/j.isci.2022.105083. eCollection 2022 Oct 21. iScience. 2022. PMID: 36147951 Free PMC article.
References
-
- BOJUNGA N, ENTIAN KD. Cat8p, the activator of gluconeogenic genes in Saccharomyces cerevisiae, regulates carbon source-dependent expression of NADP-dependent cytosolic isocitrate dehydrogenase (Idp2p) and lactate permease (Jen1p). Mol Gen Genet. 1999;262:869–75. - PubMed
-
- BRACHMANN CB, DAVIES A, COST GJ, CAPUTO E, LI J, HIETER P, BOEKE JD. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–32. - PubMed
-
- CABA E, DICKINSON DA, WARNES GR, AUBRECHT J. Differentiating mechanisms of toxicity using global gene expression analysis in Saccharomyces cerevisiae. Mutat Res. 2005;575:34–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

