Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis
- PMID: 26337719
- PMCID: PMC4559965
- DOI: 10.1186/s12916-015-0455-8
Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis
Abstract
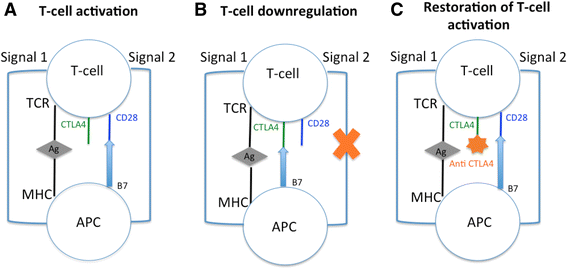
Background: Targeting CTLA-4 is a recent strategic approach in cancer control: blocking CTLA-4 enhances an antitumor immunity by promoting T-cell activation and cytotoxic T-lymphocyte proliferation. This induction of a tolerance break against the tumor may be responsible for immune-related adverse events (irAEs). Our objective was to assess the incidence and nature of irAEs in oncologic patients receiving anti-CTLA-4 antibodies (ipilimumab and tremelimumab).
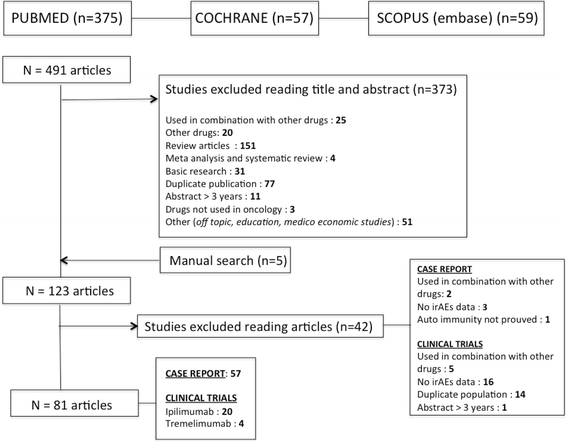
Methods: A systematic search of literature up to February 2014 was performed in MEDLINE, EMBASE, and Cochrane databases to identify relevant articles. Paired reviewers independently selected articles for inclusion and extracted data. Pooled incidence was calculated using R(©), package meta.
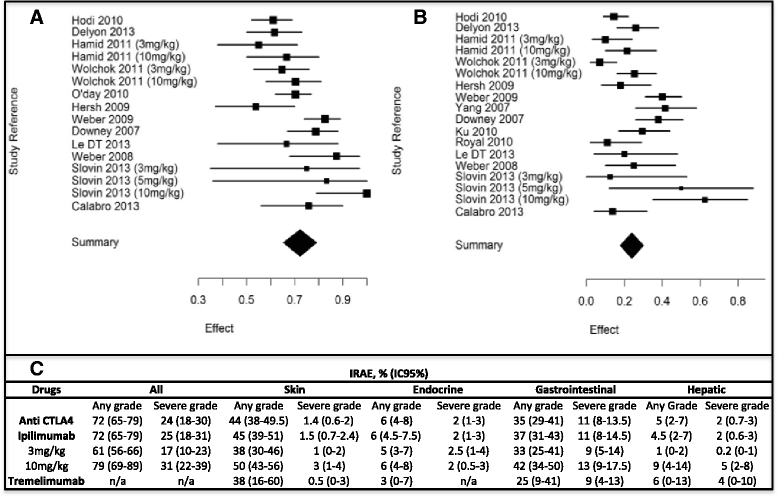
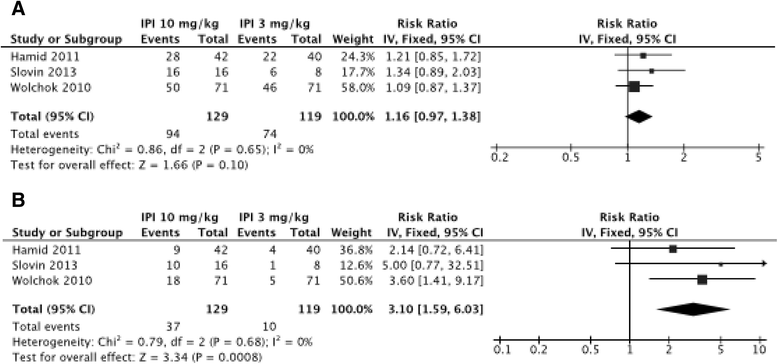
Results: Overall, 81 articles were included in the study, with a total of 1265 patients from 22 clinical trials included in the meta-analysis. Described irAEs consisted of skin lesions (rash, pruritus, and vitiligo), colitis, and less frequently hepatitis, hypophysitis, thyroiditis, and some rare events such as sarcoidosis, uveitis, Guillain-Barré syndrome, immune-mediated cytopenia and polymyalgia rheumatic/Horton. The overall incidence of all-grade irAEs was 72 % (95 % CI, 65-79 %). The overall incidence of high-grade irAEs was 24 % (95 % CI, 18-30 %). The risk of developing irAEs was dependent of dosage, with incidence of all-grade irAEs being evaluated to 61 % (95 % CI, 56-66 %) for ipilimumab 3 mg/kg and 79 % (95 % CI, 69-89 %) for ipilimumab 10 mg/kg. Death due to irAEs occurred in 0.86 % of patients. The median time of onset of irAEs was about 10 weeks (IQR, 6-12) after the onset of treatment, corresponding with the first three cycles but varied according to the organ system involved. Such immune activation could also be indicative for tumor-specific T-cell activation and irAE occurrence was associated with clinical response to CTLA-4 blocking in 60 % of patients.
Conclusion: The price of potential long-term survival to metastatic tumors is an atypical immune toxicity, reflecting the mechanism of action of anti-CTLA-4 antibodies. A better knowledge of these irAEs and its management in a multidisciplinary approach will help to reduce morbidity and therapy interruptions.
Figures
Similar articles
-
Immune-related adverse events following administration of anti-cytotoxic T-lymphocyte-associated protein-4 drugs: a comprehensive systematic review and meta-analysis.Drug Des Devel Ther. 2019 Jul 4;13:2215-2234. doi: 10.2147/DDDT.S196316. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31308633 Free PMC article.
-
Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors.J Hepatol. 2018 Jun;68(6):1181-1190. doi: 10.1016/j.jhep.2018.01.033. Epub 2018 Feb 8. J Hepatol. 2018. PMID: 29427729
-
New insight in endocrine-related adverse events associated to immune checkpoint blockade.Best Pract Res Clin Endocrinol Metab. 2020 Jan;34(1):101370. doi: 10.1016/j.beem.2019.101370. Epub 2019 Dec 11. Best Pract Res Clin Endocrinol Metab. 2020. PMID: 31983543 Review.
-
Prevalence of immune-related systemic adverse events in patients treated with anti-Programmed cell Death 1/anti-Programmed cell Death-Ligand 1 agents: A single-centre pharmacovigilance database analysis.Eur J Cancer. 2017 Sep;82:34-44. doi: 10.1016/j.ejca.2017.05.032. Epub 2017 Jul 10. Eur J Cancer. 2017. PMID: 28646772
-
Management of immune-related adverse events and kinetics of response with ipilimumab.J Clin Oncol. 2012 Jul 20;30(21):2691-7. doi: 10.1200/JCO.2012.41.6750. Epub 2012 May 21. J Clin Oncol. 2012. PMID: 22614989 Review.
Cited by
-
Immune Checkpoint Inhibitor Therapy in Oncology: Current Uses and Future Directions: JACC: CardioOncology State-of-the-Art Review.JACC CardioOncol. 2022 Dec 20;4(5):579-597. doi: 10.1016/j.jaccao.2022.09.004. eCollection 2022 Dec. JACC CardioOncol. 2022. PMID: 36636451 Free PMC article. Review.
-
Severe necrotizing myositis associated with long term anti-neoplastic efficacy following nivolumab plus ipilimumab combination therapy.Clin Rheumatol. 2019 Feb;38(2):601-602. doi: 10.1007/s10067-018-4373-y. Epub 2018 Nov 19. Clin Rheumatol. 2019. PMID: 30456528
-
Adverse events need for hospitalization and systemic immunosuppression in very elderly patients (over 80 years) treated with ipilimumab for metastatic melanoma.Cancer Immunol Immunother. 2019 Apr;68(4):545-551. doi: 10.1007/s00262-019-02298-9. Epub 2019 Jan 19. Cancer Immunol Immunother. 2019. PMID: 30661086 Free PMC article.
-
Assessment of the Novel, Practical, and Prognosis-Relevant TNM Staging System for Stage I-III Cutaneous Melanoma.Front Oncol. 2022 Apr 29;12:738298. doi: 10.3389/fonc.2022.738298. eCollection 2022. Front Oncol. 2022. PMID: 35574383 Free PMC article.
-
Advances in pathogenesis and precision medicine for nasopharyngeal carcinoma.MedComm (2020). 2021 Jan 7;2(2):175-206. doi: 10.1002/mco2.32. eCollection 2021 Jun. MedComm (2020). 2021. PMID: 34766141 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

