Slit-Dependent Endocytic Trafficking of the Robo Receptor Is Required for Son of Sevenless Recruitment and Midline Axon Repulsion
- PMID: 26335920
- PMCID: PMC4559387
- DOI: 10.1371/journal.pgen.1005402
Slit-Dependent Endocytic Trafficking of the Robo Receptor Is Required for Son of Sevenless Recruitment and Midline Axon Repulsion
Abstract
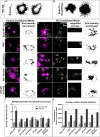
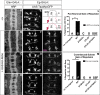
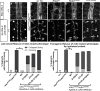
Understanding how axon guidance receptors are activated by their extracellular ligands to regulate growth cone motility is critical to learning how proper wiring is established during development. Roundabout (Robo) is one such guidance receptor that mediates repulsion from its ligand Slit in both invertebrates and vertebrates. Here we show that endocytic trafficking of the Robo receptor in response to Slit-binding is necessary for its repulsive signaling output. Dose-dependent genetic interactions and in vitro Robo activation assays support a role for Clathrin-dependent endocytosis, and entry into both the early and late endosomes as positive regulators of Slit-Robo signaling. We identify two conserved motifs in Robo's cytoplasmic domain that are required for its Clathrin-dependent endocytosis and activation in vitro; gain of function and genetic rescue experiments provide strong evidence that these trafficking events are required for Robo repulsive guidance activity in vivo. Our data support a model in which Robo's ligand-dependent internalization from the cell surface to the late endosome is essential for receptor activation and proper repulsive guidance at the midline by allowing recruitment of the downstream effector Son of Sevenless in a spatially constrained endocytic trafficking compartment.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Robo recruitment of the Wave regulatory complex plays an essential and conserved role in midline repulsion.Elife. 2021 Apr 12;10:e64474. doi: 10.7554/eLife.64474. Elife. 2021. PMID: 33843588 Free PMC article.
-
Slit Binding via the Ig1 Domain Is Essential for Midline Repulsion by Drosophila Robo1 but Dispensable for Receptor Expression, Localization, and Regulation in Vivo.G3 (Bethesda). 2015 Sep 10;5(11):2429-39. doi: 10.1534/g3.115.022327. G3 (Bethesda). 2015. PMID: 26362767 Free PMC article.
-
Drosophila neurexin IV interacts with Roundabout and is required for repulsive midline axon guidance.J Neurosci. 2010 Apr 21;30(16):5653-67. doi: 10.1523/JNEUROSCI.6187-09.2010. J Neurosci. 2010. PMID: 20410118 Free PMC article.
-
Intracellular Trafficking Mechanisms that Regulate Repulsive Axon Guidance.Neuroscience. 2023 Jan 1;508:123-136. doi: 10.1016/j.neuroscience.2022.07.012. Epub 2022 Jul 18. Neuroscience. 2023. PMID: 35863679 Free PMC article. Review.
-
Slit-Robo signaling.Development. 2016 Sep 1;143(17):3037-44. doi: 10.1242/dev.132829. Development. 2016. PMID: 27578174 Review.
Cited by
-
Cell Adhesion Molecules and Ubiquitination-Functions and Significance.Biology (Basel). 2015 Dec 23;5(1):1. doi: 10.3390/biology5010001. Biology (Basel). 2015. PMID: 26703751 Free PMC article. Review.
-
New insights into the molecular mechanisms of axon guidance receptor regulation and signaling.Curr Top Dev Biol. 2021;142:147-196. doi: 10.1016/bs.ctdb.2020.11.008. Epub 2021 Jan 18. Curr Top Dev Biol. 2021. PMID: 33706917 Free PMC article. Review.
-
A human DCC variant causing mirror movement disorder reveals that the WAVE regulatory complex mediates axon guidance by netrin-1-DCC.Sci Signal. 2024 Oct;17(856):eadk2345. doi: 10.1126/scisignal.adk2345. Epub 2024 Oct 1. Sci Signal. 2024. PMID: 39353037 Free PMC article.
-
Robo recruitment of the Wave regulatory complex plays an essential and conserved role in midline repulsion.Elife. 2021 Apr 12;10:e64474. doi: 10.7554/eLife.64474. Elife. 2021. PMID: 33843588 Free PMC article.
-
Molecular mechanisms regulating axon responsiveness at the midline.Dev Biol. 2020 Oct 1;466(1-2):12-21. doi: 10.1016/j.ydbio.2020.08.006. Epub 2020 Aug 17. Dev Biol. 2020. PMID: 32818516 Free PMC article.
References
-
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, et al. (1999) Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 96: 795–806. - PubMed
-
- Kidd T, Bland KS, Goodman CS (1999) Slit is the midline repellent for the robo receptor in Drosophila. Cell 96: 785–794. - PubMed
-
- Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, et al. (1998a) Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell 92: 205–215. - PubMed
-
- Wang KH, Brose K, Arnott D, Kidd T, Goodman CS, et al. (1999) Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell 96: 771–784. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

