Expression, purification, crystallization and X-ray diffraction studies of the molecular chaperone prefoldin from Homo sapiens
- PMID: 26323306
- PMCID: PMC4555927
- DOI: 10.1107/S2053230X15013990
Expression, purification, crystallization and X-ray diffraction studies of the molecular chaperone prefoldin from Homo sapiens
Abstract
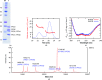
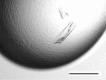
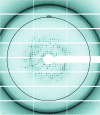
Proper protein folding is an essential process for all organisms. Prefoldin (PFD) is a molecular chaperone that assists protein folding by delivering non-native proteins to group II chaperonin. A heterohexamer of eukaryotic PFD has been shown to specifically recognize and deliver non-native actin and tubulin to chaperonin-containing TCP-1 (CCT), but the mechanism of specific recognition is still unclear. To determine its crystal structure, recombinant human PFD was reconstituted, purified and crystallized. X-ray diffraction data were collected to 4.7 Å resolution. The crystals belonged to space group P21212, with unit-cell parameters a = 123.2, b = 152.4, c = 105.9 Å.
Keywords: molecular chaperone; prefoldin; protein folding.
Figures
Similar articles
-
Comparative structural insight into prefoldin subunints of archaea and eukaryotes with special emphasis on unexplored prefoldin of Plasmodium falciparum.J Biomol Struct Dyn. 2022 May;40(8):3804-3818. doi: 10.1080/07391102.2020.1850527. Epub 2020 Dec 4. J Biomol Struct Dyn. 2022. PMID: 33272134 Review.
-
Crystallization and preliminary X-ray diffraction analysis of the beta subunit Yke2 of the Gim complex from Saccharomyces cerevisiae.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008 Jun 1;64(Pt 6):501-3. doi: 10.1107/S1744309108011846. Epub 2008 May 23. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008. PMID: 18540060 Free PMC article.
-
Structure of eukaryotic prefoldin and of its complexes with unfolded actin and the cytosolic chaperonin CCT.EMBO J. 2002 Dec 2;21(23):6377-86. doi: 10.1093/emboj/cdf640. EMBO J. 2002. PMID: 12456645 Free PMC article.
-
The Chaperonin TRiC/CCT Associates with Prefoldin through a Conserved Electrostatic Interface Essential for Cellular Proteostasis.Cell. 2019 Apr 18;177(3):751-765.e15. doi: 10.1016/j.cell.2019.03.012. Epub 2019 Apr 4. Cell. 2019. PMID: 30955883 Free PMC article.
-
Structure and Function of the Cochaperone Prefoldin.Adv Exp Med Biol. 2018;1106:119-131. doi: 10.1007/978-3-030-00737-9_9. Adv Exp Med Biol. 2018. PMID: 30484157 Review.
Cited by
-
Prefoldin Function in Cellular Protein Homeostasis and Human Diseases.Front Cell Dev Biol. 2022 Jan 17;9:816214. doi: 10.3389/fcell.2021.816214. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35111762 Free PMC article. Review.
-
Expression, Functional Characterization, and Preliminary Crystallization of the Cochaperone Prefoldin from the Thermophilic Fungus Chaetomium thermophilum.Int J Mol Sci. 2018 Aug 19;19(8):2452. doi: 10.3390/ijms19082452. Int J Mol Sci. 2018. PMID: 30126249 Free PMC article.
-
A comprehensive analysis of prefoldins and their implication in cancer.iScience. 2021 Oct 15;24(11):103273. doi: 10.1016/j.isci.2021.103273. eCollection 2021 Nov 19. iScience. 2021. PMID: 34761191 Free PMC article. Review.
-
Prefoldin, a jellyfish-like molecular chaperone: functional cooperation with a group II chaperonin and beyond.Biophys Rev. 2018 Apr;10(2):339-345. doi: 10.1007/s12551-018-0400-0. Epub 2018 Feb 9. Biophys Rev. 2018. PMID: 29427249 Free PMC article. Review.
-
Structural basis for the inhibition of IAPP fibril formation by the co-chaperonin prefoldin.Nat Commun. 2022 May 2;13(1):2363. doi: 10.1038/s41467-022-30042-y. Nat Commun. 2022. PMID: 35501361 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

