Membrane Docking of the Synaptotagmin 7 C2A Domain: Electron Paramagnetic Resonance Measurements Show Contributions from Two Membrane Binding Loops
- PMID: 26322740
- PMCID: PMC4630001
- DOI: 10.1021/acs.biochem.5b00421
Membrane Docking of the Synaptotagmin 7 C2A Domain: Electron Paramagnetic Resonance Measurements Show Contributions from Two Membrane Binding Loops
Abstract
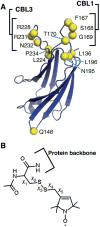
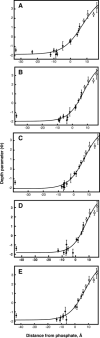
The synaptotagmin (Syt) family of proteins plays an important role in vesicle docking and fusion during Ca(2+)-induced exocytosis in a wide variety of cell types. Its role as a Ca(2+) sensor derives primarily from its two C2 domains, C2A and C2B, which insert into anionic lipid membranes upon binding Ca(2+). Syt isoforms 1 and 7 differ significantly in their Ca(2+) sensitivity; the C2A domain from Syt7 binds Ca(2+) and membranes much more tightly than the C2A domain from Syt1, at least in part because of greater contributions from the hydrophobic effect. While the structure and membrane activity of Syt1 have been extensively studied, the structural origins of differences between Syt1 and Syt7 are unknown. This study used site-directed spin labeling and electron paramagnetic resonance spectroscopy to determine depth parameters for the Syt7 C2A domain, for comparison to analogous previous measurements with the Syt1 C2A domain. In a novel approach, the membrane docking geometry of both Syt1 and Syt7 C2A was modeled by mapping depth parameters onto multiple molecular dynamics-simulated structures of the Ca(2+)-bound protein. The models reveal membrane penetration of Ca(2+) binding loops 1 (CBL1) and 3 (CBL3), and membrane binding is more sensitive to mutations in CBL3. On average, Syt7 C2A inserts more deeply into the membrane than Syt1 C2A, although depths vary among the different structural models. This observation provides a partial structural explanation for the hydrophobically driven membrane docking of Syt7 C2A.
Figures

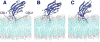
Similar articles
-
Hydrophobic contributions to the membrane docking of synaptotagmin 7 C2A domain: mechanistic contrast between isoforms 1 and 7.Biochemistry. 2012 Oct 2;51(39):7654-64. doi: 10.1021/bi3007115. Epub 2012 Sep 21. Biochemistry. 2012. PMID: 22966849 Free PMC article.
-
Membrane Docking of the Synaptotagmin 7 C2A Domain: Computation Reveals Interplay between Electrostatic and Hydrophobic Contributions.Biochemistry. 2015 Sep 22;54(37):5696-711. doi: 10.1021/acs.biochem.5b00422. Epub 2015 Sep 10. Biochemistry. 2015. PMID: 26333120
-
Exceptionally tight membrane-binding may explain the key role of the synaptotagmin-7 C2A domain in asynchronous neurotransmitter release.Proc Natl Acad Sci U S A. 2017 Oct 3;114(40):E8518-E8527. doi: 10.1073/pnas.1710708114. Epub 2017 Sep 18. Proc Natl Acad Sci U S A. 2017. PMID: 28923929 Free PMC article.
-
The C2 domains of synaptotagmin--partners in exocytosis.Trends Biochem Sci. 2004 Mar;29(3):143-51. doi: 10.1016/j.tibs.2004.01.008. Trends Biochem Sci. 2004. PMID: 15003272 Review.
-
4-[18F]Fluorobenzoyl-C2A domain of synaptotagmin I-glutathione-S-transferase.2011 May 27 [updated 2011 Aug 4]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 May 27 [updated 2011 Aug 4]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 21834184 Free Books & Documents. Review.
Cited by
-
Membrane-Binding Cooperativity and Coinsertion by C2AB Tandem Domains of Synaptotagmins 1 and 7.Biophys J. 2019 Mar 19;116(6):1025-1036. doi: 10.1016/j.bpj.2019.01.035. Epub 2019 Feb 5. Biophys J. 2019. PMID: 30795874 Free PMC article.
-
A Conserved Electrostatic Membrane-Binding Surface in Synaptotagmin-Like Proteins Revealed Using Molecular Phylogenetic Analysis and Homology Modeling.bioRxiv [Preprint]. 2023 Oct 29:2023.07.13.548768. doi: 10.1101/2023.07.13.548768. bioRxiv. 2023. Update in: Protein Sci. 2024 Jan;33(1):e4850. doi: 10.1002/pro.4850 PMID: 37502952 Free PMC article. Updated. Preprint.
-
Synaptotagmin 7 C2 domains induce membrane curvature stress via electrostatic interactions and the wedge mechanism.bioRxiv [Preprint]. 2024 Jan 12:2024.01.10.575084. doi: 10.1101/2024.01.10.575084. bioRxiv. 2024. PMID: 38313280 Free PMC article. Preprint.
-
Otoferlin C2F Domain-Induced Changes in Membrane Structure Observed by Sum Frequency Generation.Biophys J. 2019 Nov 19;117(10):1820-1830. doi: 10.1016/j.bpj.2019.09.010. Epub 2019 Sep 17. Biophys J. 2019. PMID: 31587832 Free PMC article.
-
Functions of Vertebrate Ferlins.Cells. 2020 Feb 25;9(3):534. doi: 10.3390/cells9030534. Cells. 2020. PMID: 32106631 Free PMC article. Review.
References
-
- Chapman ER. How does synaptotagmin trigger neurotransmitter release? Annu Rev Biochem. 2008;77:615–641. - PubMed
-
- Martens S, McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9:543–556. - PubMed
-
- Corbalan-Garcia S, Gomez-Fernandez JC. Signaling through C2 domains: more than one lipid target. Biochim Biophys Acta. 2014;1838:1536–1547. - PubMed
-
- Gustavsson N, Han W. Calcium-sensing beyond neurotransmitters: functions of synaptotagmins in neuroendocrine and endocrine secretion. Biosci Rep. 2009;29:245–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

