Differential N-Glycosylation Patterns in Lung Adenocarcinoma Tissue
- PMID: 26322380
- PMCID: PMC4644186
- DOI: 10.1021/acs.jproteome.5b00255
Differential N-Glycosylation Patterns in Lung Adenocarcinoma Tissue
Abstract
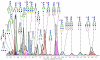
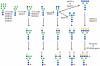
To decrease the mortality of lung cancer, better screening and diagnostic tools as well as treatment options are needed. Protein glycosylation is one of the major post-translational modifications that is altered in cancer, but it is not exactly clear which glycan structures are affected. A better understanding of the glycan structures that are differentially regulated in lung tumor tissue is highly desirable and will allow us to gain greater insight into the underlying biological mechanisms of aberrant glycosylation in lung cancer. Here, we assess differential glycosylation patterns of lung tumor tissue and nonmalignant tissue at the level of individual glycan structures using nLC-chip-TOF-MS. Using tissue samples from 42 lung adenocarcinoma patients, 29 differentially expressed (FDR < 0.05) glycan structures were identified. The levels of several oligomannose type glycans were upregulated in tumor tissue. Furthermore, levels of fully galactosylated glycans, some of which were of the hybrid type and mostly without fucose, were decreased in cancerous tissue, whereas levels of non- or low-galactosylated glycans mostly with fucose were increased. To further assess the regulation of the altered glycosylation, the glycomics data was compared to publicly available gene expression data from lung adenocarcinoma tissue compared to nonmalignant lung tissue. The results are consistent with the possibility that the observed N-glycan changes have their origin in differentially expressed glycosyltransferases. These results will be used as a starting point for the further development of clinical glycan applications in the fields of imaging, drug targeting, and biomarkers for lung cancer.
Keywords: N-Glycosylation; NSCLC; gene expression; nLC−MS profiling; tissue.
Figures

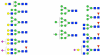
Similar articles
-
Differential N-glycan patterns identified in lung adenocarcinoma by N-glycan profiling of formalin-fixed paraffin-embedded (FFPE) tissue sections.J Proteomics. 2018 Feb 10;172:1-10. doi: 10.1016/j.jprot.2017.11.010. Epub 2017 Nov 20. J Proteomics. 2018. PMID: 29157724
-
N-glycosylation Profiling of Colorectal Cancer Cell Lines Reveals Association of Fucosylation with Differentiation and Caudal Type Homebox 1 (CDX1)/Villin mRNA Expression.Mol Cell Proteomics. 2016 Jan;15(1):124-40. doi: 10.1074/mcp.M115.051235. Epub 2015 Nov 4. Mol Cell Proteomics. 2016. PMID: 26537799 Free PMC article.
-
Identification of potential glycan cancer markers with sialic acid attached to sialic acid and up-regulated fucosylated galactose structures in epidermal growth factor receptor secreted from A431 cell line.Mol Cell Proteomics. 2013 May;12(5):1239-49. doi: 10.1074/mcp.M112.024554. Epub 2013 Jan 31. Mol Cell Proteomics. 2013. PMID: 23371026 Free PMC article.
-
Protein glycosylation in cancers and its potential therapeutic applications in neuroblastoma.J Hematol Oncol. 2016 Sep 29;9(1):100. doi: 10.1186/s13045-016-0334-6. J Hematol Oncol. 2016. PMID: 27686492 Free PMC article. Review.
-
Mass Spectrometry-Based N-Glycomics of Colorectal Cancer.Int J Mol Sci. 2015 Dec 9;16(12):29278-304. doi: 10.3390/ijms161226165. Int J Mol Sci. 2015. PMID: 26690136 Free PMC article. Review.
Cited by
-
Recent advances in N-glycan biomarker discovery among human diseases.Acta Biochim Biophys Sin (Shanghai). 2024 Jun 21;56(8):1156-1171. doi: 10.3724/abbs.2024101. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38910518 Free PMC article. Review.
-
The Utility of Differential Scanning Calorimetry Curves of Blood Plasma for Diagnosis, Subtype Differentiation and Predicted Survival in Lung Cancer.Cancers (Basel). 2021 Oct 23;13(21):5326. doi: 10.3390/cancers13215326. Cancers (Basel). 2021. PMID: 34771491 Free PMC article.
-
Proteomic profiling of lung adenocarcinoma indicates heightened DNA repair, antioxidant mechanisms and identifies LASP1 as a potential negative predictor of survival.Clin Proteomics. 2016 Oct 27;13:31. doi: 10.1186/s12014-016-9132-y. eCollection 2016. Clin Proteomics. 2016. PMID: 27799870 Free PMC article.
-
Aberrant Glycosylation in Pancreatic Ductal Adenocarcinoma 3D Organoids Is Mediated by KRAS Mutations.J Oncol. 2024 Mar 18;2024:1529449. doi: 10.1155/2024/1529449. eCollection 2024. J Oncol. 2024. PMID: 38528852 Free PMC article.
-
The Function of Fucosylation in Progression of Lung Cancer.Front Oncol. 2018 Dec 7;8:565. doi: 10.3389/fonc.2018.00565. eCollection 2018. Front Oncol. 2018. PMID: 30619732 Free PMC article. Review.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. Ca-Cancer J. Clin. 2012;62(1):10–29. - PubMed
-
- Pass HI, Beer DG, Joseph S, Massion P. Biomarkers and molecular testing for early detection, diagnosis, and therapeutic prediction of lung cancer. Thorac Surg Clin. 2013;23(2):211–24. - PubMed
-
- Packer NH, von der Lieth CW, Aoki-Kinoshita KF, Lebrilla CB, Paulson JC, Raman R, Rudd P, Sasisekharan R, Taniguchi N, York WS. Frontiers in glycomics: bioinformatics and biomarkers in disease. An NIH white paper prepared from discussions by the focus groups at a workshop on the NIH campus, Bethesda MD (September 11-13, 2006) Proteomics. 2008;8(1):8–20. - PubMed
-
- Plzak J, Holikova Z, Smetana K, Jr., Dvorankova B, Hercogova J, Kaltner H, Motlik J, Gabius HJ. Differentiation-dependent glycosylation of cells in squamous cell epithelia detected by a mammalian lectin. Cells Tissues Organs. 2002;171(2-3):135–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

