Activation of MET pathway predicts poor outcome to cetuximab in patients with recurrent or metastatic head and neck cancer
- PMID: 26319934
- PMCID: PMC4552997
- DOI: 10.1186/s12967-015-0633-7
Activation of MET pathway predicts poor outcome to cetuximab in patients with recurrent or metastatic head and neck cancer
Abstract
Background: Activation of the MET oncogene promotes tumor growth, invasion and metastasis in several tumor types. Additionally, MET is activated as a compensatory pathway in the presence of EGFR blockade, thus resulting in a mechanism of resistance to EGFR inhibitors.
Methods: We have investigated the impact of HGF and MET expression, MET activation (phosphorylation), MET gene status, and MET-activating mutations on cetuximab sensitivity in recurrent or metastatic squamous cell carcinoma of the head and neck (HNSCC) patients.
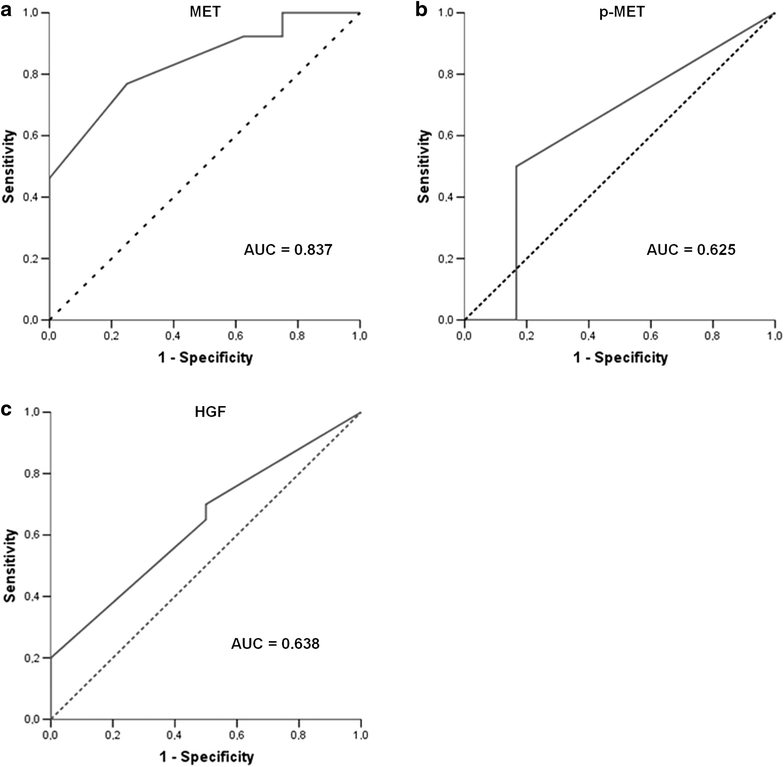
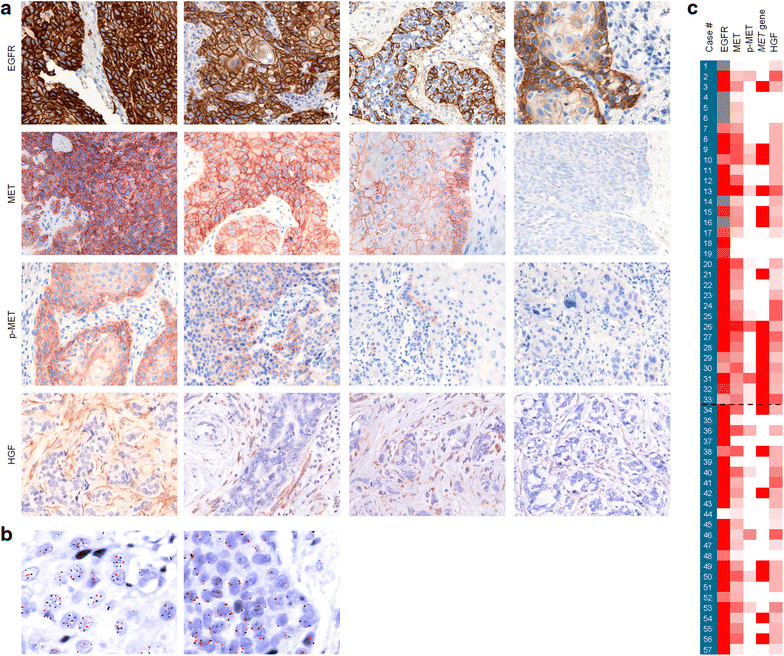
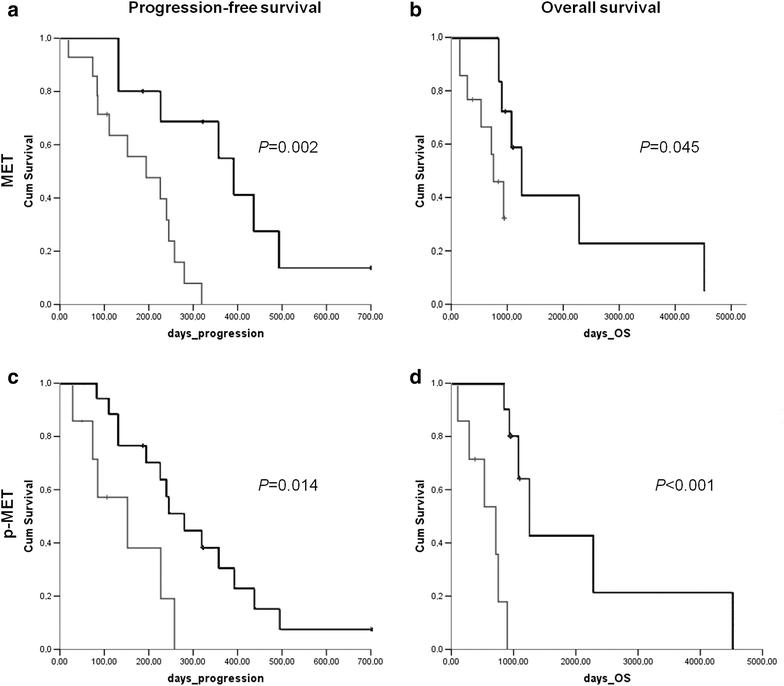
Results: A single-institution retrospective analysis was performed in 57 patients. MET overexpression was detected in 58% patients, MET amplification in 39% and MET activation (p-MET) in 30%. Amplification was associated with MET overexpression. Log-rank testing showed significantly worse outcomes in recurrent/metastatic, MET overexpressing patients for progression-free survival and overall survival. Activation of MET was correlated with worse PFS and OS. In multivariate logistic regression analysis, p-MET was an independent prognostic factor for PFS. HGF overexpression was observed in 58% patients and was associated with MET phosphorylation, suggesting a paracrine activation of the receptor.
Conclusions: HGF/MET pathway activation correlated with worse outcome in recurrent/metastatic HNSCC patients. When treated with a cetuximab-based regimen, these patients correlated with worse outcome. This supports a dual blocking strategy of HGF/MET and EGFR pathways for the treatment of patients with recurrent/metastatic HNSCC.
Figures
Similar articles
-
HGF/Met Signaling in Head and Neck Cancer: Impact on the Tumor Microenvironment.Clin Cancer Res. 2016 Aug 15;22(16):4005-13. doi: 10.1158/1078-0432.CCR-16-0951. Epub 2016 Jul 1. Clin Cancer Res. 2016. PMID: 27370607 Free PMC article. Review.
-
Is there a role for IGF1R and c-MET pathways in resistance to cetuximab in metastatic colorectal cancer?Clin Colorectal Cancer. 2011 Dec;10(4):325-32. doi: 10.1016/j.clcc.2011.03.028. Epub 2011 May 11. Clin Colorectal Cancer. 2011. PMID: 21729677
-
Prognostic role of epiregulin/amphiregulin expression in recurrent/metastatic head and neck cancer treated with cetuximab.Head Neck. 2018 Nov;40(11):2424-2431. doi: 10.1002/hed.25353. Epub 2018 Oct 10. Head Neck. 2018. PMID: 30302873
-
Comprehensive molecular analyses of lung adenocarcinoma with regard to the epidermal growth factor receptor, K-ras, MET, and hepatocyte growth factor status.J Thorac Oncol. 2010 May;5(5):591-6. doi: 10.1097/JTO.0b013e3181d0a4db. J Thorac Oncol. 2010. PMID: 20150826
-
Roles of the HGF/Met signaling in head and neck squamous cell carcinoma: Focus on tumor immunity (Review).Oncol Rep. 2020 Dec;44(6):2337-2344. doi: 10.3892/or.2020.7799. Epub 2020 Oct 9. Oncol Rep. 2020. PMID: 33125120 Review.
Cited by
-
HGF/Met Signaling in Head and Neck Cancer: Impact on the Tumor Microenvironment.Clin Cancer Res. 2016 Aug 15;22(16):4005-13. doi: 10.1158/1078-0432.CCR-16-0951. Epub 2016 Jul 1. Clin Cancer Res. 2016. PMID: 27370607 Free PMC article. Review.
-
Clinical update on head and neck cancer: molecular biology and ongoing challenges.Cell Death Dis. 2019 Jul 15;10(8):540. doi: 10.1038/s41419-019-1769-9. Cell Death Dis. 2019. PMID: 31308358 Free PMC article. Review.
-
Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial.Lancet Oncol. 2017 Nov;18(11):1467-1482. doi: 10.1016/S1470-2045(17)30566-1. Epub 2017 Sep 25. Lancet Oncol. 2017. PMID: 28958504 Free PMC article. Clinical Trial.
-
Precision Medicine Approaches to Overcome Resistance to Therapy in Head and Neck Cancers.Front Oncol. 2021 Feb 25;11:614332. doi: 10.3389/fonc.2021.614332. eCollection 2021. Front Oncol. 2021. PMID: 33718169 Free PMC article. Review.
-
Non-Invasive microRNA Profiling in Saliva can Serve as a Biomarker of Alcohol Exposure and Its Effects in Humans.Front Genet. 2022 Jan 20;12:804222. doi: 10.3389/fgene.2021.804222. eCollection 2021. Front Genet. 2022. PMID: 35126468 Free PMC article.
References
-
- Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol. 2010;21(Suppl 7):vii252–vii261. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

