Photo-thermal effect enhances the efficiency of radiotherapy using Arg-Gly-Asp peptides-conjugated gold nanorods that target αvβ3 in melanoma cancer cells
- PMID: 26315288
- PMCID: PMC4552250
- DOI: 10.1186/s12951-015-0113-5
Photo-thermal effect enhances the efficiency of radiotherapy using Arg-Gly-Asp peptides-conjugated gold nanorods that target αvβ3 in melanoma cancer cells
Abstract
Background: Thermotherapy has been known to be one of the most effective adjuvants to radiotherapy (RT) in cancer treatment, but it is not widely implemented clinically due to some limitations, such as, inadequate temperature concentrations to the tumor tissue, nonspecific and non-uniform distribution of heat. So we constructed arginine-glycine-aspartate peptides-conjugated gold nanorods (RGD-GNRs) that target the alpha(v) beta(3) Integrin (αvβ3) and investigate whether the photo-thermal effect of RGD-GNRs by near infrared radiation (NIR) could enhance the efficiency of RT in melanoma cancer cells.
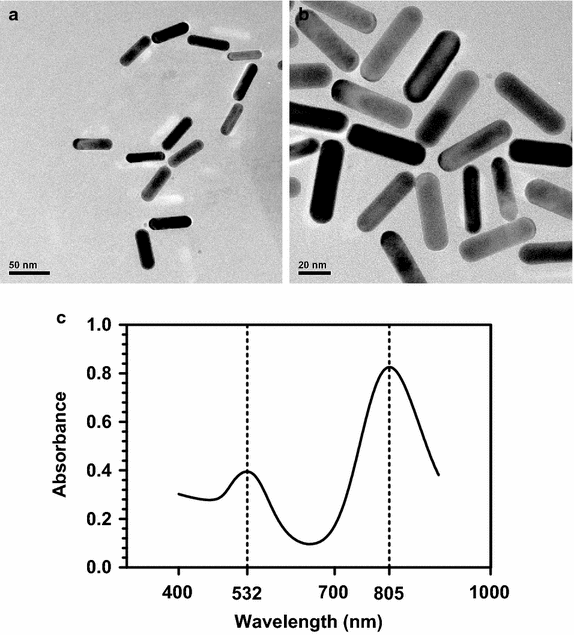
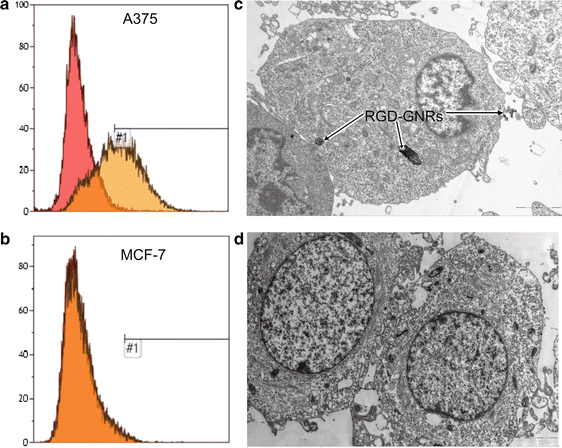
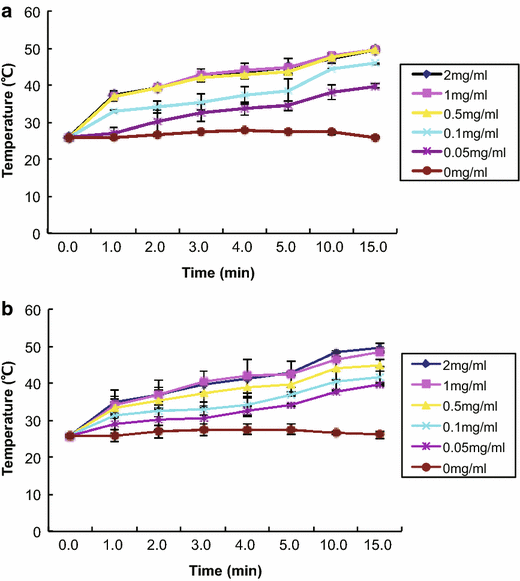
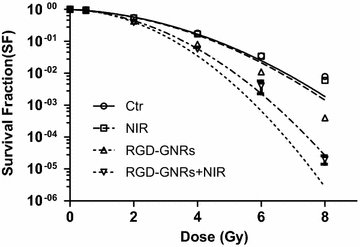
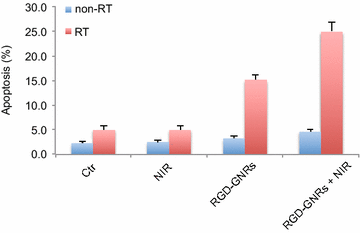
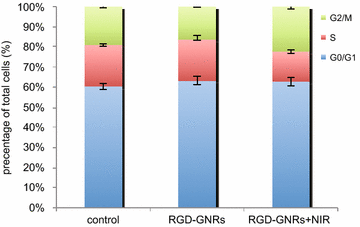
Results: RGD-GNRs could be seen both on the surface of the cell membranes and cytoplasm of A375 cells with high expression of αvβ3. After exposed to 808 nm NIR, RGD-GNRs with various concentrations could be rapidly heated up. Compared to other treatments, flow cytometric analysis indicated that RT + NIR + RGD-GNRs increased apoptosis (p < 0.001) and decreased the proportion of cells in the more radioresistant S phase (p = 0.014). Treated with NIR + RGD-GNRs, the radiosensitivity was also significantly enhanced (DMFSF2: 1.41).
Conclusion: Results of the current study showed the feasibility of using RGD-GNRs for synergetic RT with photo-thermal therapy. And it would greatly benefit the therapeutic effects of refractory or recurrent malignant cancers.
Figures
Similar articles
-
Preparation, toxicity reduction and radiation therapy application of gold nanorods.J Nanobiotechnology. 2021 Dec 28;19(1):454. doi: 10.1186/s12951-021-01209-4. J Nanobiotechnology. 2021. PMID: 34963479 Free PMC article. Review.
-
RGD-conjugated gold nanorods induce radiosensitization in melanoma cancer cells by downregulating α(v)β₃ expression.Int J Nanomedicine. 2012;7:915-24. doi: 10.2147/IJN.S28314. Epub 2012 Feb 27. Int J Nanomedicine. 2012. PMID: 22412298 Free PMC article.
-
Folic acid-conjugated silica-modified gold nanorods for X-ray/CT imaging-guided dual-mode radiation and photo-thermal therapy.Biomaterials. 2011 Dec;32(36):9796-809. doi: 10.1016/j.biomaterials.2011.08.086. Epub 2011 Sep 13. Biomaterials. 2011. PMID: 21917309
-
Investigating the effect of near infrared photo thermal therapy folic acid conjugated gold nano shell on melanoma cancer cell line A375.Artif Cells Nanomed Biotechnol. 2019 Dec;47(1):2161-2170. doi: 10.1080/21691401.2019.1593188. Artif Cells Nanomed Biotechnol. 2019. PMID: 31159585
-
Photothermal cancer therapy and imaging based on gold nanorods.Ann Biomed Eng. 2012 Feb;40(2):534-46. doi: 10.1007/s10439-011-0388-0. Epub 2011 Sep 2. Ann Biomed Eng. 2012. PMID: 21887589 Review.
Cited by
-
Preparation, toxicity reduction and radiation therapy application of gold nanorods.J Nanobiotechnology. 2021 Dec 28;19(1):454. doi: 10.1186/s12951-021-01209-4. J Nanobiotechnology. 2021. PMID: 34963479 Free PMC article. Review.
-
Application of nano-radiosensitizers in combination cancer therapy.Bioeng Transl Med. 2023 Feb 10;8(3):e10498. doi: 10.1002/btm2.10498. eCollection 2023 May. Bioeng Transl Med. 2023. PMID: 37206240 Free PMC article. Review.
-
Silk Fibroin-Modified Liposome/Gene Editing System Knocks out the PLK1 Gene to Suppress the Growth of Lung Cancer Cells.Pharmaceutics. 2023 Dec 12;15(12):2756. doi: 10.3390/pharmaceutics15122756. Pharmaceutics. 2023. PMID: 38140096 Free PMC article.
-
In vitro outlook of gold nanoparticles in photo-thermal therapy: a literature review.Lasers Med Sci. 2018 May;33(4):917-926. doi: 10.1007/s10103-018-2467-z. Epub 2018 Feb 28. Lasers Med Sci. 2018. PMID: 29492712 Review.
-
Enhancing radiotherapy for melanoma: the promise of high-Z metal nanoparticles in radiosensitization.Nanomedicine (Lond). 2024;19(28):2391-2411. doi: 10.1080/17435889.2024.2403325. Epub 2024 Oct 9. Nanomedicine (Lond). 2024. PMID: 39382020 Review.
References
-
- Wessalowski R, Schneider DT, Mils O, Friemann V, Kyrillopoulou O, Schaper J, et al. Regional deep hyperthermia for salvage treatment of children and adolescents with refractory or recurrent non-testicular malignant germ-cell tumours: an open-label, non-randomised, single-institution, phase 2 study. Lancet Oncol. 2013;14:843–852. doi: 10.1016/S1470-2045(13)70271-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

