Synapse-specific IL-1 receptor subunit reconfiguration augments vulnerability to IL-1β in the aged hippocampus
- PMID: 26305968
- PMCID: PMC4568670
- DOI: 10.1073/pnas.1514486112
Synapse-specific IL-1 receptor subunit reconfiguration augments vulnerability to IL-1β in the aged hippocampus
Abstract
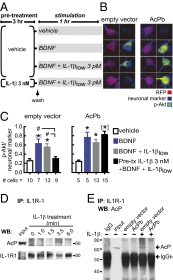
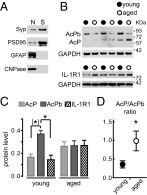
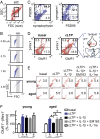
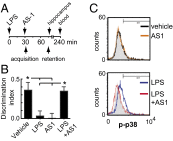
In the aged brain, synaptic plasticity and memory show increased vulnerability to impairment by the inflammatory cytokine interleukin 1β (IL-1β). In this study, we evaluated the possibility that synapses may directly undergo maladaptive changes with age that augment sensitivity to IL-1β impairment. In hippocampal neuronal cultures, IL-1β increased the expression of the IL-1 receptor type 1 and the accessory coreceptor AcP (proinflammatory), but not of the AcPb (prosurvival) subunit, a reconfiguration that potentiates the responsiveness of neurons to IL-1β. To evaluate whether synapses develop a similar heightened sensitivity to IL-1β with age, we used an assay to track long-term potentiation (LTP) in synaptosomes. We found that IL-1β impairs LTP directly at the synapse and that sensitivity to IL-1β is augmented in aged hippocampal synapses. The increased synaptic sensitivity to IL-1β was due to IL-1 receptor subunit reconfiguration, characterized by a shift in the AcP/AcPb ratio, paralleling our culture data. We suggest that the age-related increase in brain IL-1β levels drives a shift in IL-1 receptor configuration, thus heightening the sensitivity to IL-1β. Accordingly, selective blocking of AcP-dependent signaling with Toll-IL-1 receptor domain peptidomimetics prevented IL-1β-mediated LTP suppression and blocked the memory impairment induced in aged mice by peripheral immune challenge (bacterial lipopolysaccharide). Overall, this study demonstrates that increased AcP signaling, specifically at the synapse, underlies the augmented vulnerability to cognitive impairment by IL-1β that occurs with age.
Keywords: AcP; AcPb; LTP; neuroinflammation; receptor sensitivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
TNFα and IL-1β but not IL-18 Suppresses Hippocampal Long-Term Potentiation Directly at the Synapse.Neurochem Res. 2019 Jan;44(1):49-60. doi: 10.1007/s11064-018-2517-8. Epub 2018 Apr 4. Neurochem Res. 2019. PMID: 29619614 Free PMC article.
-
Neuron-specific effects of interleukin-1β are mediated by a novel isoform of the IL-1 receptor accessory protein.J Neurosci. 2011 Dec 7;31(49):18048-59. doi: 10.1523/JNEUROSCI.4067-11.2011. J Neurosci. 2011. PMID: 22159118 Free PMC article.
-
Brain-specific interleukin-1 receptor accessory protein in sleep regulation.J Appl Physiol (1985). 2012 Mar;112(6):1015-22. doi: 10.1152/japplphysiol.01307.2011. Epub 2011 Dec 15. J Appl Physiol (1985). 2012. PMID: 22174404 Free PMC article.
-
Immune dysregulation and cognitive vulnerability in the aging brain: Interactions of microglia, IL-1β, BDNF and synaptic plasticity.Neuropharmacology. 2015 Sep;96(Pt A):11-8. doi: 10.1016/j.neuropharm.2014.12.020. Epub 2014 Dec 27. Neuropharmacology. 2015. PMID: 25549562 Free PMC article. Review.
-
BDNF-induced local protein synthesis and synaptic plasticity.Neuropharmacology. 2014 Jan;76 Pt C:639-56. doi: 10.1016/j.neuropharm.2013.04.005. Epub 2013 Apr 16. Neuropharmacology. 2014. PMID: 23602987 Review.
Cited by
-
Gastrodin Ameliorates Cognitive Dysfunction in Diabetes Rat Model via the Suppression of Endoplasmic Reticulum Stress and NLRP3 Inflammasome Activation.Front Pharmacol. 2018 Nov 22;9:1346. doi: 10.3389/fphar.2018.01346. eCollection 2018. Front Pharmacol. 2018. PMID: 30524286 Free PMC article.
-
Young and aged TLR4 deficient mice show sex-dependent enhancements in spatial memory and alterations in interleukin-1 related genes.Brain Behav Immun. 2019 Feb;76:37-47. doi: 10.1016/j.bbi.2018.10.010. Epub 2018 Oct 27. Brain Behav Immun. 2019. PMID: 30394314 Free PMC article.
-
Structural basis of the IL-1 receptor TIR domain-mediated IL-1 signaling.iScience. 2022 Jun 2;25(7):104508. doi: 10.1016/j.isci.2022.104508. eCollection 2022 Jul 15. iScience. 2022. PMID: 35754719 Free PMC article.
-
Inflammation: the link between comorbidities, genetics, and Alzheimer's disease.J Neuroinflammation. 2018 Sep 24;15(1):276. doi: 10.1186/s12974-018-1313-3. J Neuroinflammation. 2018. PMID: 30249283 Free PMC article. Review.
-
Functional role of brain-engrafted macrophages against brain injuries.J Neuroinflammation. 2021 Oct 15;18(1):232. doi: 10.1186/s12974-021-02290-0. J Neuroinflammation. 2021. PMID: 34654458 Free PMC article.
References
-
- Lynch MA. Age-related impairment in long-term potentiation in hippocampus: A role for the cytokine, interleukin-1 beta? Prog Neurobiol. 1998;56(5):571–589. - PubMed
-
- Trompet S, et al. PROSPER Group Genetic variation in the interleukin-1 beta-converting enzyme associates with cognitive function. The PROSPER study. Brain. 2008;131(Pt 4):1069–1077. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

