Resveratrol compounds inhibit human holocarboxylase synthetase and cause a lean phenotype in Drosophila melanogaster
- PMID: 26303405
- PMCID: PMC4631641
- DOI: 10.1016/j.jnutbio.2015.07.004
Resveratrol compounds inhibit human holocarboxylase synthetase and cause a lean phenotype in Drosophila melanogaster
Abstract
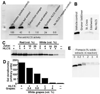
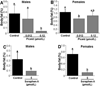
Holocarboxylase synthetase (HLCS) is the sole protein-biotin ligase in the human proteome. HLCS has key regulatory functions in intermediary metabolism, including fatty acid metabolism, and in gene repression through epigenetic mechanisms. The objective of this study was to identify food-borne inhibitors of HLCS that alter HLCS-dependent pathways in metabolism and gene regulation. When libraries of extracts from natural products and chemically pure compounds were screened for HLCS inhibitor activity, resveratrol compounds in grape materials caused an HLCS inhibition of >98% in vitro. The potency of these compounds was piceatannol>resveratrol>piceid. Grape-borne compounds other than resveratrol metabolites also contributed toward HLCS inhibition, e.g., p-coumaric acid and cyanidin chloride. HLCS inhibitors had meaningful effects on body fat mass. When Drosophila melanogaster brummer mutants, which are genetically predisposed to storing excess amounts of lipids, were fed diets enriched with grape leaf extracts and piceid, body fat mass decreased by more than 30% in males and females. However, Drosophila responded to inhibitor treatment with an increase in the expression of HLCS, which elicited an increase in the abundance of biotinylated carboxylases in vivo. We conclude that mechanisms other than inhibition of HLCS cause body fat loss in flies. We propose that the primary candidate is the inhibition of the insulin receptor/Akt signaling pathway.
Keywords: Drosophila; Fat mass; Grapes; Holocarboxylase synthetase; Inhibitor; Resveratrol compounds.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Three promoters regulate the transcriptional activity of the human holocarboxylase synthetase gene.J Nutr Biochem. 2013 Nov;24(11):1963-9. doi: 10.1016/j.jnutbio.2013.06.007. Epub 2013 Sep 26. J Nutr Biochem. 2013. PMID: 24075901 Free PMC article.
-
Differential growth inhibition, cell cycle arrest and apoptosis of MCF-7 and MDA-MB-231 cells to holocarboxylase synthetase suppression.Biochem Biophys Res Commun. 2022 Feb 19;593:108-115. doi: 10.1016/j.bbrc.2022.01.049. Epub 2022 Jan 14. Biochem Biophys Res Commun. 2022. PMID: 35063765
-
β-Keto and β-hydroxyphosphonate analogs of biotin-5'-AMP are inhibitors of holocarboxylase synthetase.Bioorg Med Chem Lett. 2014 Dec 15;24(24):5568-5571. doi: 10.1016/j.bmcl.2014.11.010. Epub 2014 Nov 7. Bioorg Med Chem Lett. 2014. PMID: 25466176 Free PMC article.
-
Novel roles of holocarboxylase synthetase in gene regulation and intermediary metabolism.Nutr Rev. 2014 Jun;72(6):369-76. doi: 10.1111/nure.12103. Epub 2014 Mar 28. Nutr Rev. 2014. PMID: 24684412 Free PMC article. Review.
-
Regulation of immunological and inflammatory functions by biotin.Can J Physiol Pharmacol. 2015 Dec;93(12):1091-6. doi: 10.1139/cjpp-2014-0460. Epub 2015 Mar 26. Can J Physiol Pharmacol. 2015. PMID: 26168302 Review.
Cited by
-
The Therapeutic Potential of Piceatannol, a Natural Stilbene, in Metabolic Diseases: A Review.J Med Food. 2017 May;20(5):427-438. doi: 10.1089/jmf.2017.3916. Epub 2017 Apr 7. J Med Food. 2017. PMID: 28387565 Free PMC article. Review.
References
-
- Zempleni J, Wijeratne SSK, Kuroishi T. Biotin. In: Erdman JW Jr, Macdonald I, Zeisel SH, editors. Present Knowledge in Nutrition. Washington, D.C.: International Life Sciences Institute; 2012. pp. 587–609.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

