Innate and Adaptive Immune Responses in Chronic HCV Infection
- PMID: 26302811
- PMCID: PMC5625838
- DOI: 10.2174/1389450116666150825110532
Innate and Adaptive Immune Responses in Chronic HCV Infection
Abstract
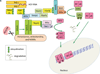
Hepatitis C virus (HCV) remains a public health problem of global importance, even in the era of potent directly-acting antiviral drugs. In this chapter, I discuss immune responses to acute and chronic HCV infection. The outcome of HCV infection is influenced by viral strategies that limit or delay the initiation of innate antiviral responses. This delay may enable HCV to establish widespread infection long before the host mounts effective T and B cell responses. HCV's genetic agility, resulting from its high rate of replication and its error prone replication mechanism, enables it to evade immune recognition. Adaptive immune responses fail to keep up with changing viral epitopes. Neutralizing antibody epitopes may be hidden by decoy structures, glycans, and lipoproteins. T cell responses fail due to changing epitope sequences and due to exhaustion, a phenomenon that may have evolved to limit immune-mediated pathology. Despite these difficulties, innate and adaptive immune mechanisms do impact HCV replication. Immune-mediated clearance of infection is possible, occurring in 20-50% of people who contract the disease. New developments raise hopes for effective immunological interventions to prevent or treat HCV infection.
Keywords: Hepatitis C virus; T cell exhaustion; immune response; innate immunity; interferon lambda; neutralizing antibodies.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Figures



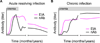
Similar articles
-
Innate and adaptive immune responses in HCV infections.J Hepatol. 2014 Nov;61(1 Suppl):S14-25. doi: 10.1016/j.jhep.2014.06.035. Epub 2014 Nov 3. J Hepatol. 2014. PMID: 25443342 Review.
-
Flying under the radar: the immunobiology of hepatitis C.Annu Rev Immunol. 2007;25:71-99. doi: 10.1146/annurev.immunol.25.022106.141602. Annu Rev Immunol. 2007. PMID: 17067278 Review.
-
Pathogen-Associated Molecular Pattern Recognition of Hepatitis C Virus Transmitted/Founder Variants by RIG-I Is Dependent on U-Core Length.J Virol. 2015 Nov;89(21):11056-68. doi: 10.1128/JVI.01964-15. Epub 2015 Aug 26. J Virol. 2015. PMID: 26311867 Free PMC article.
-
Immune and non-immune responses to hepatitis C virus infection.World J Gastroenterol. 2015 Oct 14;21(38):10739-48. doi: 10.3748/wjg.v21.i38.10739. World J Gastroenterol. 2015. PMID: 26478666 Free PMC article. Review.
-
Failure of innate and adaptive immune responses in controlling hepatitis C virus infection.FEMS Microbiol Rev. 2012 May;36(3):663-83. doi: 10.1111/j.1574-6976.2011.00319.x. Epub 2012 Jan 4. FEMS Microbiol Rev. 2012. PMID: 22142141 Review.
Cited by
-
Efficient acute and chronic infection of stem cell-derived hepatocytes by hepatitis C virus.Gut. 2020 Sep;69(9):1659-1666. doi: 10.1136/gutjnl-2019-319354. Epub 2020 Feb 29. Gut. 2020. PMID: 32114504 Free PMC article.
-
HCV-related liver and lymphoproliferative diseases: association with polymorphisms of IL28B and TLR2.Oncotarget. 2016 Jun 21;7(25):37487-37497. doi: 10.18632/oncotarget.9303. Oncotarget. 2016. PMID: 27183918 Free PMC article.
-
Innate Immune Response against Hepatitis C Virus: Targets for Vaccine Adjuvants.Vaccines (Basel). 2020 Jun 17;8(2):313. doi: 10.3390/vaccines8020313. Vaccines (Basel). 2020. PMID: 32560440 Free PMC article. Review.
-
Prevalence and Outcome of Serum Autoantibodies in Chronic Hepatitis C Patients Undergoing Direct-Acting Antiviral Treatment.Front Immunol. 2022 Apr 11;13:882064. doi: 10.3389/fimmu.2022.882064. eCollection 2022. Front Immunol. 2022. PMID: 35479086 Free PMC article.
-
Evaluating the Therapeutic Potential of Durvalumab in Adults with Locally Advanced or Metastatic Biliary Tract Cancer: Evidence to Date.Onco Targets Ther. 2024 May 17;17:383-394. doi: 10.2147/OTT.S391707. eCollection 2024. Onco Targets Ther. 2024. PMID: 38774819 Free PMC article. Review.
References
-
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10(9):553–562. - PubMed
-
- Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17(2):107–115. - PubMed
-
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–1342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

