Tbx15 controls skeletal muscle fibre-type determination and muscle metabolism
- PMID: 26299309
- PMCID: PMC4552045
- DOI: 10.1038/ncomms9054
Tbx15 controls skeletal muscle fibre-type determination and muscle metabolism
Abstract
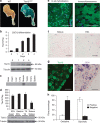
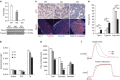
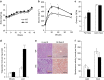
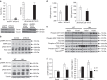
Skeletal muscle is composed of both slow-twitch oxidative myofibers and fast-twitch glycolytic myofibers that differentially impact muscle metabolism, function and eventually whole-body physiology. Here we show that the mesodermal transcription factor T-box 15 (Tbx15) is highly and specifically expressed in glycolytic myofibers. Ablation of Tbx15 in vivo leads to a decrease in muscle size due to a decrease in the number of glycolytic fibres, associated with a small increase in the number of oxidative fibres. This shift in fibre composition results in muscles with slower myofiber contraction and relaxation, and also decreases whole-body oxygen consumption, reduces spontaneous activity, increases adiposity and glucose intolerance. Mechanistically, ablation of Tbx15 leads to activation of AMPK signalling and a decrease in Igf2 expression. Thus, Tbx15 is one of a limited number of transcription factors to be identified with a critical role in regulating glycolytic fibre identity and muscle metabolism.
Figures
Similar articles
-
Tbx15 Defines a Glycolytic Subpopulation and White Adipocyte Heterogeneity.Diabetes. 2017 Nov;66(11):2822-2829. doi: 10.2337/db17-0218. Epub 2017 Aug 28. Diabetes. 2017. PMID: 28847884 Free PMC article.
-
AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity.FASEB J. 2014 Jul;28(7):3211-24. doi: 10.1096/fj.14-250449. Epub 2014 Mar 20. FASEB J. 2014. PMID: 24652947
-
Genesis of muscle fiber-type diversity during mouse embryogenesis relies on Six1 and Six4 gene expression.Dev Biol. 2011 Nov 15;359(2):303-20. doi: 10.1016/j.ydbio.2011.08.010. Epub 2011 Aug 22. Dev Biol. 2011. PMID: 21884692
-
Relationship between muscle fibre composition, glucose transporter protein 4 and exercise training: possible consequences in non-insulin-dependent diabetes mellitus.Acta Physiol Scand. 2001 Mar;171(3):267-76. doi: 10.1046/j.1365-201x.2001.00829.x. Acta Physiol Scand. 2001. PMID: 11412139 Review.
-
AMPK regulation of fatty acid metabolism and mitochondrial biogenesis: implications for obesity.Mol Cell Endocrinol. 2013 Feb 25;366(2):135-51. doi: 10.1016/j.mce.2012.06.019. Epub 2012 Jun 28. Mol Cell Endocrinol. 2013. PMID: 22750049 Review.
Cited by
-
Cell autonomous requirement of neurofibromin (Nf1) for postnatal muscle hypertrophic growth and metabolic homeostasis.J Cachexia Sarcopenia Muscle. 2020 Dec;11(6):1758-1778. doi: 10.1002/jcsm.12632. Epub 2020 Oct 19. J Cachexia Sarcopenia Muscle. 2020. PMID: 33078583 Free PMC article.
-
Integrative analysis deciphers the heterogeneity of cancer-associated fibroblast and implications on clinical outcomes in ovarian cancers.Comput Struct Biotechnol J. 2022 Nov 14;20:6403-6411. doi: 10.1016/j.csbj.2022.11.025. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36420154 Free PMC article.
-
Dynamic enhancers control skeletal muscle identity and reprogramming.PLoS Biol. 2019 Oct 7;17(10):e3000467. doi: 10.1371/journal.pbio.3000467. eCollection 2019 Oct. PLoS Biol. 2019. PMID: 31589602 Free PMC article.
-
Enhanced exercise and regenerative capacity in a mouse model that violates size constraints of oxidative muscle fibres.Elife. 2016 Aug 5;5:e16940. doi: 10.7554/eLife.16940. Elife. 2016. PMID: 27494364 Free PMC article.
-
Insulin and IGF-1 receptors regulate FoxO-mediated signaling in muscle proteostasis.J Clin Invest. 2016 Sep 1;126(9):3433-46. doi: 10.1172/JCI86522. Epub 2016 Aug 15. J Clin Invest. 2016. PMID: 27525440 Free PMC article.
References
-
- He J., Watkins S. & Kelley D. E. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes 50, 817 (2001). - PubMed
-
- Hickey M. S. et al. Skeletal muscle fiber composition is related to adiposity and in vitro glucose transport rate in humans. Am. J. Physiol. 268, E453–E457 (1995). - PubMed
-
- Nyholm B. et al. Evidence of an increased number of type IIb muscle fibers in insulin-resistant first-degree relatives of patients with NIDDM. Diabetes 46, 1822 (1997). - PubMed
-
- Tanner C. J. et al. Muscle fiber type is associated with obesity and weight loss. Am. J. Physiol. Endocrinol. Metab. 282, E1191–E1196 (2002). - PubMed
-
- Marin P., Andersson B., Krotkiewski M. & Bjorntorp P. Muscle fiber composition and capillary density in women and men with NIDDM. Diabetes Care 17, 382 (1994). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

