Intracellular amyloid β oligomers impair organelle transport and induce dendritic spine loss in primary neurons
- PMID: 26293809
- PMCID: PMC4546183
- DOI: 10.1186/s40478-015-0230-2
Intracellular amyloid β oligomers impair organelle transport and induce dendritic spine loss in primary neurons
Erratum in
-
Erratum: Intracellular amyloid β oligomers impair organelle transport and induce dendritic spine loss in primary neurons.Acta Neuropathol Commun. 2016 Jan 28;4:7. doi: 10.1186/s40478-016-0273-z. Acta Neuropathol Commun. 2016. PMID: 26822851 Free PMC article.
Abstract
Introduction: Synaptic dysfunction and intracellular transport defects are early events in Alzheimer's disease (AD). Extracellular amyloid β (Aβ) oligomers cause spine alterations and impede the transport of proteins and organelles such as brain-derived neurotrophic factor (BDNF) and mitochondria that are required for synaptic function. Meanwhile, intraneuronal accumulation of Aβ precedes its extracellular deposition and is also associated with synaptic dysfunction in AD. However, the links between intracellular Aβ, spine alteration, and mechanisms that support synaptic maintenance such as organelle trafficking are poorly understood.
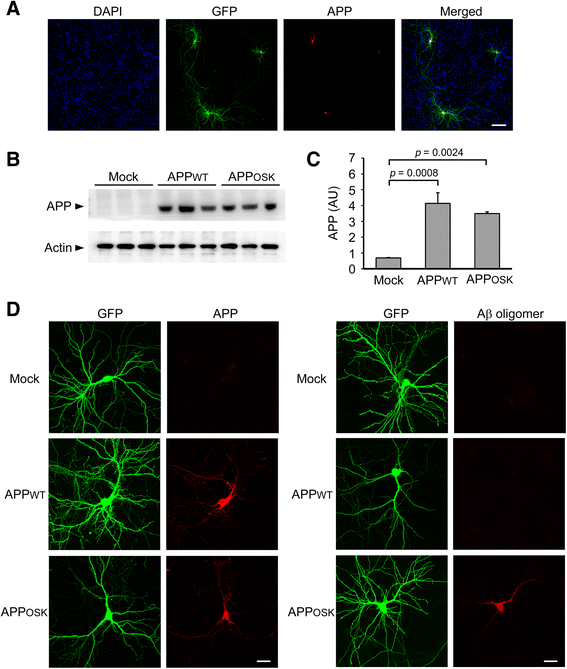
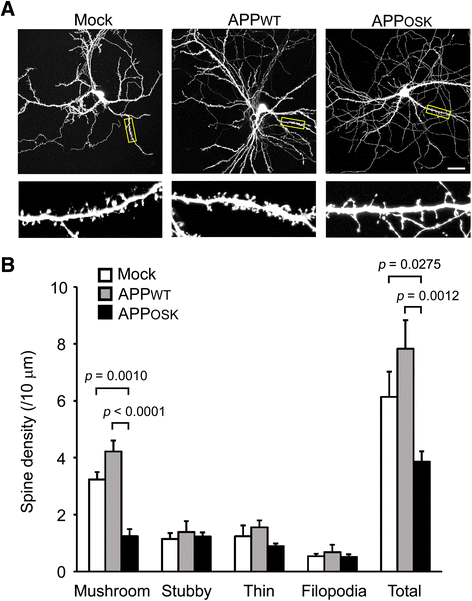
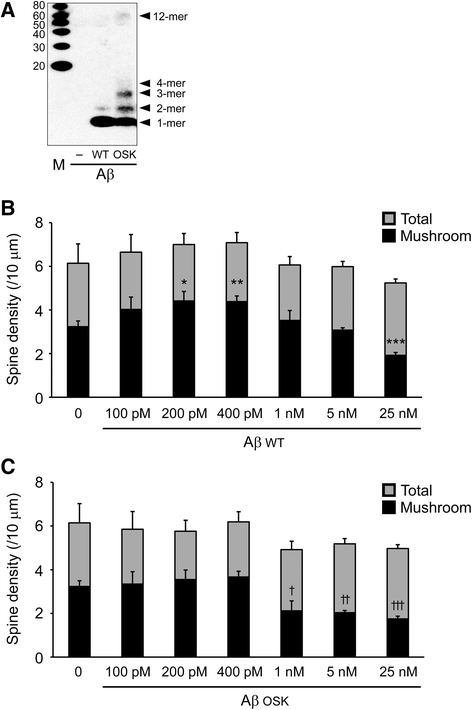
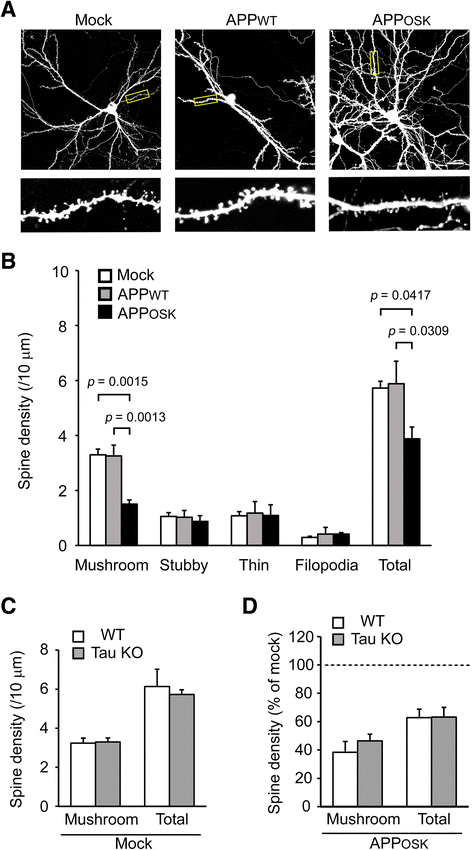
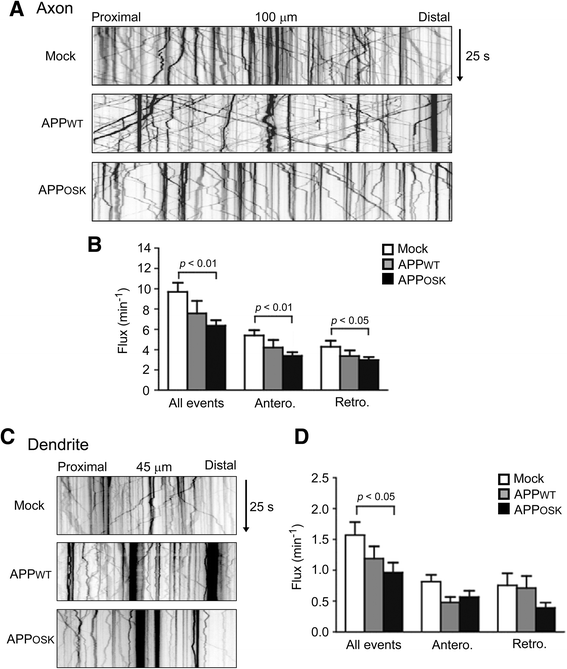
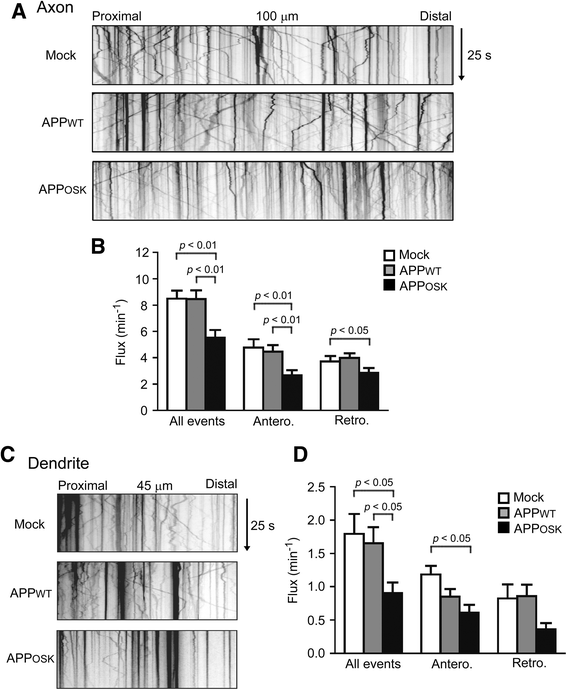
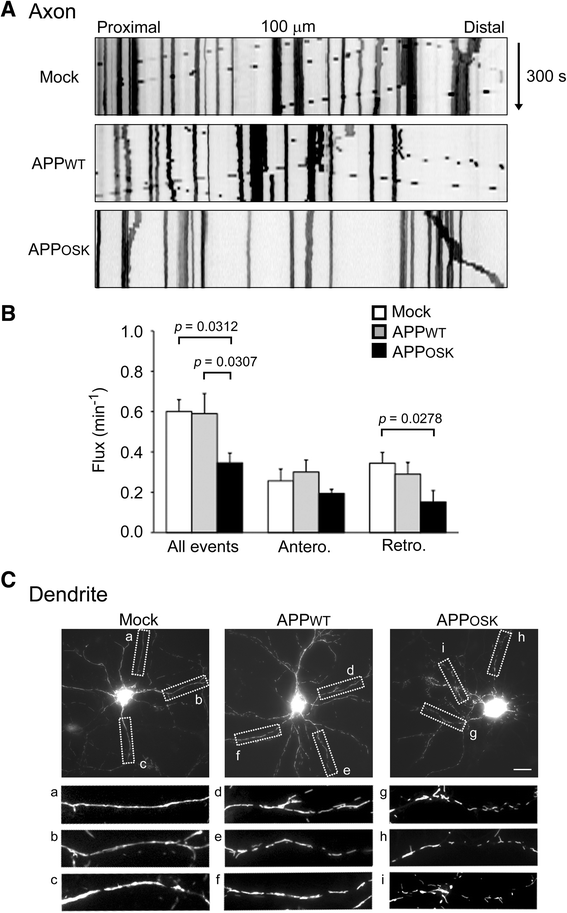
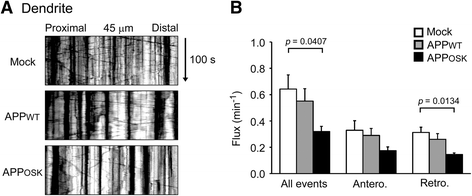
Results: We compared the effects of wild-type and Osaka (E693Δ)-mutant amyloid precursor proteins: the former secretes Aβ into extracellular space and the latter accumulates Aβ oligomers within cells. First we investigated the effects of intracellular Aβ oligomers on dendritic spines in primary neurons and their tau-dependency using tau knockout neurons. We found that intracellular Aβ oligomers caused a reduction in mushroom, or mature spines, independently of tau. We also found that intracellular Aβ oligomers significantly impaired the intracellular transport of BDNF, mitochondria, and recycling endosomes: cargoes essential for synaptic maintenance. A reduction in BDNF transport by intracellular Aβ oligomers was also observed in tau knockout neurons.
Conclusions: Our findings indicate that intracellular Aβ oligomers likely contribute to early synaptic pathology in AD and argue against the consensus that Aβ-induced spine loss and transport defects require tau.
Figures
Similar articles
-
Intracellular accumulation of toxic turn amyloid-β is associated with endoplasmic reticulum stress in Alzheimer's disease.Curr Alzheimer Res. 2013 Jan;10(1):11-20. Curr Alzheimer Res. 2013. PMID: 22950910
-
Linking amyloid-β and tau: amyloid-β induced synaptic dysfunction via local wreckage of the neuronal cytoskeleton.Neurodegener Dis. 2012;10(1-4):64-72. doi: 10.1159/000332816. Epub 2011 Dec 7. Neurodegener Dis. 2012. PMID: 22156588
-
Intraneuronal APP and extracellular Aβ independently cause dendritic spine pathology in transgenic mouse models of Alzheimer's disease.Acta Neuropathol. 2015 Jun;129(6):909-20. doi: 10.1007/s00401-015-1421-4. Epub 2015 Apr 11. Acta Neuropathol. 2015. PMID: 25862638 Free PMC article.
-
Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria, and synaptic deprivation in Alzheimer's disease.Brain Res. 2011 Sep 30;1415:136-48. doi: 10.1016/j.brainres.2011.07.052. Epub 2011 Jul 31. Brain Res. 2011. PMID: 21872849 Free PMC article. Review.
-
Thin, stubby or mushroom: spine pathology in Alzheimer's disease.Curr Alzheimer Res. 2009 Jun;6(3):261-8. doi: 10.2174/156720509788486554. Curr Alzheimer Res. 2009. PMID: 19519307 Review.
Cited by
-
Neuronal Cell Death.Physiol Rev. 2018 Apr 1;98(2):813-880. doi: 10.1152/physrev.00011.2017. Physiol Rev. 2018. PMID: 29488822 Free PMC article. Review.
-
Amyloid Pathology in the Central Auditory Pathway of 5XFAD Mice Appears First in Auditory Cortex.J Alzheimers Dis. 2022;89(4):1385-1402. doi: 10.3233/JAD-220538. J Alzheimers Dis. 2022. PMID: 36031901 Free PMC article.
-
In vitro prion-like behaviour of TDP-43 in ALS.Neurobiol Dis. 2016 Dec;96:236-247. doi: 10.1016/j.nbd.2016.08.007. Epub 2016 Aug 30. Neurobiol Dis. 2016. PMID: 27590623 Free PMC article.
-
Intracellular Trafficking Mechanisms of Synaptic Dysfunction in Alzheimer's Disease.Front Cell Neurosci. 2020 Apr 17;14:72. doi: 10.3389/fncel.2020.00072. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32362813 Free PMC article.
-
Transcranial Electromagnetic Treatment Stops Alzheimer's Disease Cognitive Decline over a 2½-Year Period: A Pilot Study.Medicines (Basel). 2022 Aug 3;9(8):42. doi: 10.3390/medicines9080042. Medicines (Basel). 2022. PMID: 36005647 Free PMC article.
References
-
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. - DOI - PMC - PubMed
-
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

