Netrin1-DCC-Mediated Attraction Guides Post-Crossing Commissural Axons in the Hindbrain
- PMID: 26290247
- PMCID: PMC4540804
- DOI: 10.1523/JNEUROSCI.0613-15.2015
Netrin1-DCC-Mediated Attraction Guides Post-Crossing Commissural Axons in the Hindbrain
Abstract
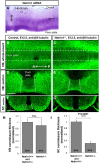
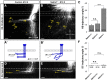
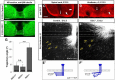
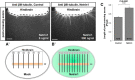
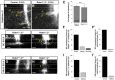
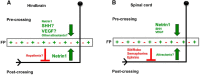
Commissural axons grow along precise trajectories that are guided by several cues secreted from the ventral midline. After initial attraction to the floor plate using Netrin1 activation of its main attractive receptor, DCC (deleted in colorectal cancer), axons cross the ventral midline, and many turn to grow longitudinally on the contralateral side. After crossing the midline, axons are thought to lose their responsiveness to Netrin1 and become sensitive to midline Slit-Robo repulsion. We aimed to address the in vivo significance of Netrin1 in guiding post-crossing axon trajectories in mouse embryos. Surprisingly, in contrast to the spinal cord, Netrin1 and DCC mutants had abundant commissural axons crossing in the hindbrain. In Netrin1 and DCC mutants, many post-crossing axons made normal turns to grow longitudinally, but projected abnormally at angles away from the midline. In addition, exposure of cultured hindbrain explants to ectopic Netrin1 caused attractive deflection of post-crossing axons. Thus, Netrin1-DCC signaling is not required to attract pre-crossing axons toward the hindbrain floor plate, but is active in post-crossing guidance. Also in contrast with spinal cord, analysis of hindbrain post-crossing axons in Robo1/2 mutant embryos showed that Slit-Robo repulsive signaling was not required for post-crossing trajectories. Our findings show that Netrin1-DCC attractive signaling, but not Slit-Robo repulsive signaling, remains active in hindbrain post-crossing commissural axons to guide longitudinal trajectories, suggesting surprising regional diversity in commissural axon guidance mechanisms.
Significance statement: The left and right sides of the brainstem and spinal cord are connected primarily by axon fibers that grow across the ventral midline, and then away on the other side to their targets. Based on spinal cord, axons are initially attracted by diffusible attractive protein signals to approach and cross the midline, and then are thought to switch to repulsive cues to grow away on the opposite side. Our results in the hindbrain show that the major midline attractant, Netrin1, is not required for midline crossing. However, the post-crossing axons depend on Netrin1 attraction to set their proper trajectories on the other side. Overall, these findings suggest that commissural axons use distinct mechanisms to navigate in different CNS regions.
Keywords: DCC; Netrin; Robo; Slit; axon guidance; commissural axon.
Copyright © 2015 the authors 0270-6474/15/3511707-12$15.00/0.
Figures

Similar articles
-
Pioneer midbrain longitudinal axons navigate using a balance of Netrin attraction and Slit repulsion.Neural Dev. 2014 Jul 24;9:17. doi: 10.1186/1749-8104-9-17. Neural Dev. 2014. PMID: 25056828 Free PMC article.
-
Motor neuron cell bodies are actively positioned by Slit/Robo repulsion and Netrin/DCC attraction.Dev Biol. 2015 Mar 1;399(1):68-79. doi: 10.1016/j.ydbio.2014.12.014. Epub 2014 Dec 18. Dev Biol. 2015. PMID: 25530182 Free PMC article.
-
A tug of war between DCC and ROBO1 signaling during commissural axon guidance.Cell Rep. 2023 May 30;42(5):112455. doi: 10.1016/j.celrep.2023.112455. Epub 2023 May 5. Cell Rep. 2023. PMID: 37149867 Free PMC article.
-
Axon guidance at the midline choice point.Dev Dyn. 2001 Jun;221(2):154-81. doi: 10.1002/dvdy.1143. Dev Dyn. 2001. PMID: 11376484 Review.
-
Netrin, Slit and Wnt receptors allow axons to choose the axis of migration.Dev Biol. 2008 Nov 15;323(2):143-51. doi: 10.1016/j.ydbio.2008.08.027. Epub 2008 Sep 5. Dev Biol. 2008. PMID: 18801355 Review.
Cited by
-
Houshiheisan and its components promote axon regeneration after ischemic brain injury.Neural Regen Res. 2018 Jul;13(7):1195-1203. doi: 10.4103/1673-5374.235031. Neural Regen Res. 2018. PMID: 30028327 Free PMC article.
-
Axonal Projection Patterns of the Dorsal Interneuron Populations in the Embryonic Hindbrain.Front Neuroanat. 2021 Dec 24;15:793161. doi: 10.3389/fnana.2021.793161. eCollection 2021. Front Neuroanat. 2021. PMID: 35002640 Free PMC article. Review.
-
Motor neuron migration and positioning mechanisms: New roles for guidance cues.Semin Cell Dev Biol. 2019 Jan;85:78-83. doi: 10.1016/j.semcdb.2017.11.016. Epub 2017 Nov 14. Semin Cell Dev Biol. 2019. PMID: 29141180 Free PMC article. Review.
-
Smoothened overexpression causes trochlear motoneurons to reroute and innervate ipsilateral eyes.Cell Tissue Res. 2021 Apr;384(1):59-72. doi: 10.1007/s00441-020-03352-0. Epub 2021 Jan 6. Cell Tissue Res. 2021. PMID: 33409653 Free PMC article.
-
Contralateral migration of oculomotor neurons is regulated by Slit/Robo signaling.Neural Dev. 2016 Oct 22;11(1):18. doi: 10.1186/s13064-016-0073-y. Neural Dev. 2016. PMID: 27770832 Free PMC article.
References
-
- Bielle F, Marcos-Mondéjar P, Leyva-Díaz E, Lokmane L, Mire E, Mailhes C, Keita M, García N, Tessier-Lavigne M, Garel S, López-Bendito G. Emergent growth cone responses to combinations of Slit1 and Netrin 1 in thalamocortical axon topography. Curr Biol. 2011;21:1748–1755. doi: 10.1016/j.cub.2011.09.008. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 RR016464/RR/NCRR NIH HHS/United States
- NS077169/NS/NINDS NIH HHS/United States
- R01 HD038069/HD/NICHD NIH HHS/United States
- R01 NS054740/NS/NINDS NIH HHS/United States
- 1 P20 RR024210/RR/NCRR NIH HHS/United States
- NS054740/NS/NINDS NIH HHS/United States
- P20 GM103440/GM/NIGMS NIH HHS/United States
- 1 P20 GM103650/GM/NIGMS NIH HHS/United States
- R01 EY025205/EY/NEI NIH HHS/United States
- P20 GM103650/GM/NIGMS NIH HHS/United States
- P20 GM103440-11/GM/NIGMS NIH HHS/United States
- R21 NS077169/NS/NINDS NIH HHS/United States
- P20 GM103554/GM/NIGMS NIH HHS/United States
- HD38069/HD/NICHD NIH HHS/United States
- P20 RR024210/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
