Vascular smooth muscle cell functional contractility depends on extracellular mechanical properties
- PMID: 26283412
- PMCID: PMC4592799
- DOI: 10.1016/j.jbiomech.2015.07.029
Vascular smooth muscle cell functional contractility depends on extracellular mechanical properties
Abstract
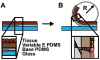
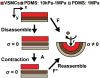
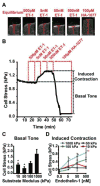
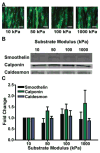
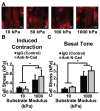
Vascular smooth muscle cells' primary function is to maintain vascular homeostasis through active contraction and relaxation. In diseases such as hypertension and atherosclerosis, this function is inhibited concurrent to changes in the mechanical environment surrounding vascular smooth muscle cells. It is well established that cell function and extracellular mechanics are interconnected; variations in substrate modulus affect cell migration, proliferation, and differentiation. To date, it is unknown how the evolving extracellular mechanical environment of vascular smooth muscle cells affects their contractile function. Here, we have built upon previous vascular muscular thin film technology to develop a variable-modulus vascular muscular thin film that measures vascular tissue functional contractility on substrates with a range of pathological and physiological moduli. Using this modified vascular muscular thin film, we found that vascular smooth muscle cells generated greater stress on substrates with higher moduli compared to substrates with lower moduli. We then measured protein markers typically thought to indicate a contractile phenotype in vascular smooth muscle cells and found that phenotype is unaffected by substrate modulus. These data suggest that mechanical properties of vascular smooth muscle cells' extracellular environment directly influence their functional behavior and do so without inducing phenotype switching.
Keywords: Arterial mechanics; Atherosclerosis; Extracellular matrix; Hypertension; Vascular muscular thin film.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Figures


Similar articles
-
Vascular smooth muscle contractility depends on cell shape.Integr Biol (Camb). 2011 Nov;3(11):1063-70. doi: 10.1039/c1ib00061f. Epub 2011 Oct 12. Integr Biol (Camb). 2011. PMID: 21993765 Free PMC article.
-
The Phenotypic Responses of Vascular Smooth Muscle Cells Exposed to Mechanical Cues.Cells. 2021 Aug 26;10(9):2209. doi: 10.3390/cells10092209. Cells. 2021. PMID: 34571858 Free PMC article. Review.
-
The effects of matrix stiffness and RhoA on the phenotypic plasticity of smooth muscle cells in a 3-D biosynthetic hydrogel system.Biomaterials. 2008 Jun;29(17):2597-607. doi: 10.1016/j.biomaterials.2008.02.005. Epub 2008 Mar 14. Biomaterials. 2008. PMID: 18342366 Free PMC article.
-
Extracellular matrix presentation modulates vascular smooth muscle cell mechanotransduction.Matrix Biol. 2015 Jan;41:36-43. doi: 10.1016/j.matbio.2014.11.001. Epub 2014 Nov 15. Matrix Biol. 2015. PMID: 25448408
-
Biomimetic control of vascular smooth muscle cell morphology and phenotype for functional tissue-engineered small-diameter blood vessels.J Biomed Mater Res A. 2009 Mar 15;88(4):1104-21. doi: 10.1002/jbm.a.32318. J Biomed Mater Res A. 2009. PMID: 19097157 Review.
Cited by
-
Oscillatory fluid-induced mechanobiology in heart valves with parallels to the vasculature.Vasc Biol. 2020 Feb 17;2(1):R59-R71. doi: 10.1530/VB-19-0031. eCollection 2020. Vasc Biol. 2020. PMID: 32923975 Free PMC article. Review.
-
Mimicking the Tendon Microenvironment to Enhance Skeletal Muscle Adhesion and Longevity in a Functional Microcantilever Platform.ACS Biomater Sci Eng. 2023 Aug 14;9(8):4698-4708. doi: 10.1021/acsbiomaterials.3c00235. Epub 2023 Jul 18. ACS Biomater Sci Eng. 2023. PMID: 37462389 Free PMC article.
-
Drugst.One - A plug-and-play solution for online systems medicine and network-based drug repurposing.ArXiv [Preprint]. 2023 Jul 4:arXiv:2305.15453v2. ArXiv. 2023. Update in: Nucleic Acids Res. 2024 Jul 5;52(W1):W481-W488. doi: 10.1093/nar/gkae388. PMID: 37332567 Free PMC article. Updated. Preprint.
-
Multiphoton Imaging of Collagen, Elastin, and Calcification in Intact Soft-Tissue Samples.Curr Protoc Cytom. 2019 Jan;87(1):e51. doi: 10.1002/cpcy.51. Epub 2018 Oct 31. Curr Protoc Cytom. 2019. PMID: 30379412 Free PMC article.
-
Biomechanics-mediated endocytosis in atherosclerosis.Front Cardiovasc Med. 2024 Apr 4;11:1337679. doi: 10.3389/fcvm.2024.1337679. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38638885 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

