Associations between deepness of response and clinical outcomes among Japanese patients with metastatic colorectal cancer treated with second-line FOLFIRI plus cetuximab
- PMID: 26273206
- PMCID: PMC4532210
- DOI: 10.2147/OTT.S87101
Associations between deepness of response and clinical outcomes among Japanese patients with metastatic colorectal cancer treated with second-line FOLFIRI plus cetuximab
Abstract
Background: In the FIRE-3 trial, overall survival (OS) was significantly longer in patients treated with FOLFIRI plus cetuximab (C-mab) than in those treated with FOLFIRI plus bevacizumab (Bev), but progression-free survival (PFS) was not significantly different. This may be associated with the deepness of response (DpR) in patients treated with FOLFIRI plus C-mab. We aimed to evaluate the relationship between clinical outcome and DpR in metastatic colorectal cancer (mCRC) patients treated with second-line FOLFIRI plus C-mab.
Methods: A total of 112 patients with histopathologically confirmed mCRC treated with second-line FOLFIRI in combination with C-mab (N=42) or Bev (N=70) were retrospectively enrolled between October 2008 and June 2013. The relationship between DpR and clinical outcome in patients treated with FOLFIRI plus C-mab or Bev was determined.
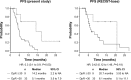
Results: Forty-two patients treated with FOLFIRI plus C-mab had a mean DpR of 6.1% (inter-quartile range: -13.7%, 20.8%) and a minimum DpR of -62.7%. On the other hand, 70 patients treated with FOLFIRI plus Bev had a mean DpR of 0% (interquartile range: -16%, 10%) and a minimum DpR of -111%. DpR ≥30% was associated with significantly longer OS and PFS when compared with DpR ≤30% in patients given FOLFIRI plus C-mab. DpR (≥30%) was independently associated with prolongation of OS and PFS. In patients treated with FOLFIRI plus C-mab, there was a moderate positive correlation between DpR and clinical outcomes (OS: r=0.51, P<0.001; PFS: r=0.54, P<0.001).
Conclusion: FOLFIRI plus C-mab yielded a stronger correlation between DpR and clinical outcomes. These results indicate the potential of DpR as a new measure of efficacy in mCRC patients treated with second-line chemotherapy plus C-mab.
Keywords: cetuximab; deepness of response; metastatic colorectal cancer; second-line chemotherapy.
Figures


Similar articles
-
'Deepness of Response' Is Associated with Overall Survival in Standard Systemic Chemotherapy for Metastatic Colorectal Cancer.Chemotherapy. 2014;60(5-6):360-7. doi: 10.1159/000438941. Epub 2015 Sep 22. Chemotherapy. 2014. PMID: 26389969
-
Early Tumor Shrinkage and Depth of Response in the Second-Line Treatment for KRAS exon2 Wild-Type Metastatic Colorectal Cancer: An Exploratory Analysis of the Randomized Phase 2 Trial Comparing Panitumumab and Bevacizumab in Combination with FOLFIRI (WJOG6210G).Target Oncol. 2020 Oct;15(5):623-633. doi: 10.1007/s11523-020-00750-w. Target Oncol. 2020. PMID: 32960408 Clinical Trial.
-
Relevance of baseline carcinoembryonic antigen for first-line treatment against metastatic colorectal cancer with FOLFIRI plus cetuximab or bevacizumab (FIRE-3 trial).Eur J Cancer. 2019 Jan;106:115-125. doi: 10.1016/j.ejca.2018.10.001. Epub 2018 Nov 27. Eur J Cancer. 2019. PMID: 30496943
-
Phase II Randomized Trial of Sequential or Concurrent FOLFOXIRI-Bevacizumab Versus FOLFOX-Bevacizumab for Metastatic Colorectal Cancer (STEAM).Oncologist. 2019 Jul;24(7):921-932. doi: 10.1634/theoncologist.2018-0344. Epub 2018 Dec 14. Oncologist. 2019. PMID: 30552157 Free PMC article. Clinical Trial.
-
FOLFOX plus anti-epidermal growth factor receptor (EGFR) monoclonal antibody (mAb) is an effective first-line treatment for patients with RAS-wild left-sided metastatic colorectal cancer: A meta-analysis.Medicine (Baltimore). 2018 Mar;97(10):e0097. doi: 10.1097/MD.0000000000010097. Medicine (Baltimore). 2018. PMID: 29517682 Free PMC article. Review.
Cited by
-
A narrative review: depth of response as a predictor of the long-term outcomes for solid tumors.Transl Cancer Res. 2021 Feb;10(2):1119-1130. doi: 10.21037/tcr-20-2547. Transl Cancer Res. 2021. PMID: 35116438 Free PMC article. Review.
-
Modified two-dimensional response as surrogate marker of overall survival in patients with metastatic colorectal cancer.Cancer Sci. 2016 Oct;107(10):1492-1498. doi: 10.1111/cas.13023. Cancer Sci. 2016. PMID: 27479846 Free PMC article.
References
-
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;20(25(12)):1539–1544. - PubMed
-
- Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE) J Clin Oncol. 2008;26(33):5326–5334. - PubMed
-
- Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147) a randomized phase 3 trial. Lancet Oncol. 2013;14(1):29–37. - PubMed
-
- Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: Phase III Trial of Cetuximab Plus Irinotecan After Fluoropyrimidine and Oxaliplatin Failure in Patients With Metastatic Colorectal Cancer. J Clin Oncol. 2008;26(14):2311–2319. - PubMed
-
- Peeters M, Price TJ, Cervantes A, et al. Randomized Phase III Study of Panitumumab With Fluorouracil, Leucovorin, and Irinotecan (FOLFIRI) Compared With FOLFIRI Alone As Second-Line Treatment in Patients With Metastatic Colorectal Cancer. J Clin Oncol. 2010;28(31):4706–4713. - PubMed
LinkOut - more resources
Full Text Sources

