High Resolution Discovery Proteomics Reveals Candidate Disease Progression Markers of Alzheimer's Disease in Human Cerebrospinal Fluid
- PMID: 26270474
- PMCID: PMC4535975
- DOI: 10.1371/journal.pone.0135365
High Resolution Discovery Proteomics Reveals Candidate Disease Progression Markers of Alzheimer's Disease in Human Cerebrospinal Fluid
Abstract
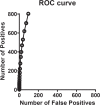
Disease modifying treatments for Alzheimer's disease (AD) constitute a major goal in medicine. Current trends suggest that biomarkers reflective of AD neuropathology and modifiable by treatment would provide supportive evidence for disease modification. Nevertheless, a lack of quantitative tools to assess disease modifying treatment effects remains a major hurdle. Cerebrospinal fluid (CSF) biochemical markers such as total tau, p-tau and Ab42 are well established markers of AD; however, global quantitative biochemical changes in CSF in AD disease progression remain largely uncharacterized. Here we applied a high resolution open discovery platform, dMS, to profile a cross-sectional cohort of lumbar CSF from post-mortem diagnosed AD patients versus those from non-AD/non-demented (control) patients. Multiple markers were identified to be statistically significant in the cohort tested. We selected two markers SME-1 (p<0.0001) and SME-2 (p = 0.0004) for evaluation in a second independent longitudinal cohort of human CSF from post-mortem diagnosed AD patients and age-matched and case-matched control patients. In cohort-2, SME-1, identified as neuronal secretory protein VGF, and SME-2, identified as neuronal pentraxin receptor-1 (NPTXR), in AD were 21% (p = 0.039) and 17% (p = 0.026) lower, at baseline, respectively, than in controls. Linear mixed model analysis in the longitudinal cohort estimate a decrease in the levels of VGF and NPTXR at the rate of 10.9% and 6.9% per year in the AD patients, whereas both markers increased in controls. Because these markers are detected by mass spectrometry without the need for antibody reagents, targeted MS based assays provide a clear translation path for evaluating selected AD disease-progression markers with high analytical precision in the clinic.
Conflict of interest statement
Figures

Similar articles
-
Cerebrospinal fluid neuronal pentraxin receptor as a biomarker of long-term progression of Alzheimer's disease: a 24-month follow-up study.Neurobiol Aging. 2020 Sep;93:97.e1-97.e7. doi: 10.1016/j.neurobiolaging.2020.03.013. Epub 2020 Mar 31. Neurobiol Aging. 2020. PMID: 32362369
-
A Parallel Reaction Monitoring Mass Spectrometric Method for Analysis of Potential CSF Biomarkers for Alzheimer's Disease.Proteomics Clin Appl. 2018 Jan;12(1). doi: 10.1002/prca.201700131. Epub 2017 Nov 23. Proteomics Clin Appl. 2018. PMID: 29028155
-
Quantitative Proteomic Profiling of Cerebrospinal Fluid to Identify Candidate Biomarkers for Alzheimer's Disease.Proteomics Clin Appl. 2019 Jul;13(4):e1800105. doi: 10.1002/prca.201800105. Epub 2019 Jan 25. Proteomics Clin Appl. 2019. PMID: 30578620 Free PMC article.
-
Simultaneous analysis of cerebrospinal fluid biomarkers using microsphere-based xMAP multiplex technology for early detection of Alzheimer's disease.Methods. 2012 Apr;56(4):484-93. doi: 10.1016/j.ymeth.2012.03.023. Epub 2012 Apr 6. Methods. 2012. PMID: 22503777 Review.
-
Cerebrospinal fluid tau, A beta, and phosphorylated tau protein for the diagnosis of Alzheimer's disease.J Cell Physiol. 2006 Jul;208(1):39-46. doi: 10.1002/jcp.20602. J Cell Physiol. 2006. PMID: 16447254 Review.
Cited by
-
Association between serum NPTX2 and cognitive function in patients with vascular dementia.Brain Behav. 2020 Oct;10(10):e01779. doi: 10.1002/brb3.1779. Epub 2020 Aug 3. Brain Behav. 2020. PMID: 32748547 Free PMC article.
-
The VGF-derived Peptide TLQP21 Impairs Purinergic Control of Chemotaxis and Phagocytosis in Mouse Microglia.J Neurosci. 2020 Apr 22;40(17):3320-3331. doi: 10.1523/JNEUROSCI.1458-19.2020. Epub 2020 Feb 14. J Neurosci. 2020. PMID: 32060170 Free PMC article.
-
Neuronal pentraxins as biomarkers of synaptic activity: from physiological functions to pathological changes in neurodegeneration.J Neural Transm (Vienna). 2022 Feb;129(2):207-230. doi: 10.1007/s00702-021-02411-2. Epub 2021 Aug 30. J Neural Transm (Vienna). 2022. PMID: 34460014 Free PMC article. Review.
-
Biological Significance of the Protein Changes Occurring in the Cerebrospinal Fluid of Alzheimer's Disease Patients: Getting Clues from Proteomic Studies.Diagnostics (Basel). 2021 Sep 9;11(9):1655. doi: 10.3390/diagnostics11091655. Diagnostics (Basel). 2021. PMID: 34573996 Free PMC article. Review.
-
Interrogation of the human cortical peptidome uncovers cell-type specific signatures of cognitive resilience against Alzheimer's disease.Sci Rep. 2024 Mar 26;14(1):7161. doi: 10.1038/s41598-024-57104-z. Sci Rep. 2024. PMID: 38531951 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

