Exchange Protein Directly Activated by cAMP (EPAC) Regulates Neuronal Polarization through Rap1B
- PMID: 26269639
- PMCID: PMC6605123
- DOI: 10.1523/JNEUROSCI.3645-14.2015
Exchange Protein Directly Activated by cAMP (EPAC) Regulates Neuronal Polarization through Rap1B
Abstract
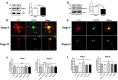
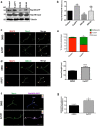
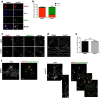
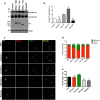
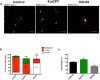
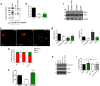
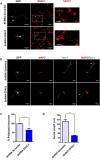
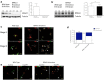
Acquisition of neuronal polarity is a complex process involving cellular and molecular events. The second messenger cAMP is involved in axonal specification through activation of protein kinase A. However, an alternative cAMP-dependent mechanism involves the exchange protein directly activated by cAMP (EPAC), which also responds to physiological changes in cAMP concentration, promoting activation of the small Rap GTPases. Here, we present evidence that EPAC signaling contributes to axon specification and elongation. In primary rat hippocampal neurons, EPAC isoforms were expressed differentially during axon specification. Furthermore, 8-pCPT, an EPAC pharmacological activator, and genetic manipulations of EPAC in neurons induced supernumerary axons indicative of Rap1b activation. Moreover, 8-pCPT-treated neurons expressed ankyrin G and other markers of mature axons such as synaptophysin and axonal accumulation of vGLUT1. In contrast, pharmacological inhibition of EPAC delayed neuronal polarity. Genetic manipulations to inactivate EPAC1 using either shRNA or neurons derived from EPAC1 knock-out (KO) mice led to axon elongation and polarization defects. Interestingly, multiaxonic neurons generated by 8-pCPT treatments in wild-type neurons were not found in EPAC1 KO mice neurons. Altogether, these results propose that EPAC signaling is an alternative and complementary mechanism for cAMP-dependent axon determination.
Significance statement: This study identifies the guanine exchange factor responsible for Rap1b activation during neuronal polarization and provides an alternate explanation for cAMP-dependent acquisition of neuronal polarity.
Keywords: EPAC signaling; Rap1b signaling; axon; axon initial segment; cytoskeleton; neuronal polarity.
Copyright © 2015 the authors 0270-6474/15/3511315-15$15.00/0.
Figures
Similar articles
-
Longest neurite-specific activation of Rap1B in hippocampal neurons contributes to polarity formation through RalA and Nore1A in addition to PI3-kinase.Genes Cells. 2013 Nov;18(11):1020-31. doi: 10.1111/gtc.12097. Epub 2013 Oct 6. Genes Cells. 2013. PMID: 24165023
-
Tuba Activates Cdc42 during Neuronal Polarization Downstream of the Small GTPase Rab8a.J Neurosci. 2021 Feb 24;41(8):1636-1649. doi: 10.1523/JNEUROSCI.0633-20.2020. Epub 2021 Jan 21. J Neurosci. 2021. PMID: 33478991 Free PMC article.
-
A novel cyclic AMP-dependent Epac-Rit signaling pathway contributes to PACAP38-mediated neuronal differentiation.Mol Cell Biol. 2006 Dec;26(23):9136-47. doi: 10.1128/MCB.00332-06. Epub 2006 Sep 25. Mol Cell Biol. 2006. PMID: 17000774 Free PMC article.
-
Rap-linked cAMP signaling Epac proteins: compartmentation, functioning and disease implications.Cell Signal. 2011 Aug;23(8):1257-66. doi: 10.1016/j.cellsig.2011.03.007. Epub 2011 Mar 22. Cell Signal. 2011. PMID: 21402149 Review.
-
Cell physiology of cAMP sensor Epac.J Physiol. 2006 Nov 15;577(Pt 1):5-15. doi: 10.1113/jphysiol.2006.119644. Epub 2006 Sep 14. J Physiol. 2006. PMID: 16973695 Free PMC article. Review.
Cited by
-
AKAP-mediated feedback control of cAMP gradients in developing hippocampal neurons.Nat Chem Biol. 2017 Apr;13(4):425-431. doi: 10.1038/nchembio.2298. Epub 2017 Feb 13. Nat Chem Biol. 2017. PMID: 28192412 Free PMC article.
-
Glucose-dependent insulinotropic polypeptide (GIP) alleviates ferroptosis in aging-induced brain damage through the Epac/Rap1 signaling pathway.BMB Rep. 2024 Sep;57(9):417-423. doi: 10.5483/BMBRep.2024-0067. BMB Rep. 2024. PMID: 39219045 Free PMC article.
-
How Rap and its GEFs control liver physiology and cancer development. C3G alterations in human hepatocarcinoma.Hepat Oncol. 2018 Apr 16;5(1):HEP05. doi: 10.2217/hep-2017-0026. eCollection 2018 Jan. Hepat Oncol. 2018. PMID: 30302196 Free PMC article. Review.
-
Heterogeneity of the Axon Initial Segment in Interneurons and Pyramidal Cells of Rodent Visual Cortex.Front Cell Neurosci. 2017 Nov 6;11:332. doi: 10.3389/fncel.2017.00332. eCollection 2017. Front Cell Neurosci. 2017. PMID: 29170630 Free PMC article.
-
Photoactivated adenylyl cyclase (PAC) reveals novel mechanisms underlying cAMP-dependent axonal morphogenesis.Sci Rep. 2016 Jan 22;5:19679. doi: 10.1038/srep19679. Sci Rep. 2016. PMID: 26795422 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
