Correlates of Vaccine-Induced Protection against Mycobacterium tuberculosis Revealed in Comparative Analyses of Lymphocyte Populations
- PMID: 26269537
- PMCID: PMC4580742
- DOI: 10.1128/CVI.00301-15
Correlates of Vaccine-Induced Protection against Mycobacterium tuberculosis Revealed in Comparative Analyses of Lymphocyte Populations
Abstract
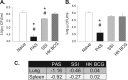
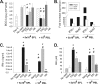
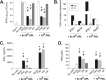
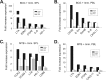
A critical hindrance to the development of a novel vaccine against Mycobacterium tuberculosis is a lack of understanding of protective correlates of immunity and of host factors involved in a successful adaptive immune response. Studies from our group and others have used a mouse-based in vitro model system to assess correlates of protection. Here, using this coculture system and a panel of whole-cell vaccines with varied efficacy, we developed a comprehensive approach to understand correlates of protection. We compared the gene and protein expression profiles of vaccine-generated immune peripheral blood lymphocytes (PBLs) to the profiles found in immune splenocytes. PBLs not only represent a clinically relevant cell population, but comparing the expression in these populations gave insight into compartmentally specific mechanisms of protection. Additionally, we performed a direct comparison of host responses induced when immune cells were cocultured with either the vaccine strain Mycobacterium bovis BCG or virulent M. tuberculosis. These comparisons revealed host-specific and bacterium-specific factors involved in protection against virulent M. tuberculosis. Most significantly, we identified a set of 13 core molecules induced in the most protective vaccines under all of the conditions tested. Further validation of this panel of mediators as a predictor of vaccine efficacy will facilitate vaccine development, and determining how each promotes adaptive immunity will advance our understanding of antimycobacterial immune responses.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
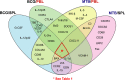
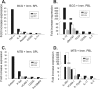
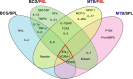
Similar articles
-
Enhanced protection against tuberculosis by vaccination with recombinant Mycobacterium microti vaccine that induces T cell immunity against region of difference 1 antigens.J Infect Dis. 2004 Jul 1;190(1):115-22. doi: 10.1086/421468. Epub 2004 Jun 4. J Infect Dis. 2004. PMID: 15195250
-
A live attenuated BCG vaccine overexpressing multistage antigens Ag85B and HspX provides superior protection against Mycobacterium tuberculosis infection.Appl Microbiol Biotechnol. 2015 Dec;99(24):10587-95. doi: 10.1007/s00253-015-6962-x. Epub 2015 Sep 12. Appl Microbiol Biotechnol. 2015. PMID: 26363555
-
Lack of immune responses to Mycobacterium tuberculosis DosR regulon proteins following Mycobacterium bovis BCG vaccination.Infect Immun. 2007 Jul;75(7):3523-30. doi: 10.1128/IAI.01999-06. Epub 2007 May 14. Infect Immun. 2007. PMID: 17502400 Free PMC article.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
-
Tuberculosis vaccine types and timings.Clin Vaccine Immunol. 2015 Mar;22(3):249-57. doi: 10.1128/CVI.00718-14. Epub 2014 Dec 24. Clin Vaccine Immunol. 2015. PMID: 25540272 Free PMC article. Review.
Cited by
-
Working correlates of protection predict SchuS4-derived-vaccine candidates with improved efficacy against an intracellular bacterium, Francisella tularensis.NPJ Vaccines. 2022 Aug 17;7(1):95. doi: 10.1038/s41541-022-00506-9. NPJ Vaccines. 2022. PMID: 35977964 Free PMC article.
-
A panel of correlates predicts vaccine-induced protection of rats against respiratory challenge with virulent Francisella tularensis.PLoS One. 2018 May 25;13(5):e0198140. doi: 10.1371/journal.pone.0198140. eCollection 2018. PLoS One. 2018. PMID: 29799870 Free PMC article.
-
Immune lymphocytes halt replication of Francisella tularensis LVS within the cytoplasm of infected macrophages.Sci Rep. 2020 Jul 21;10(1):12023. doi: 10.1038/s41598-020-68798-2. Sci Rep. 2020. PMID: 32694562 Free PMC article.
-
Bacterial Vaccine Antigen Discovery in the Reverse Vaccinology 2.0 Era: Progress and Challenges.Front Immunol. 2018 Oct 8;9:2315. doi: 10.3389/fimmu.2018.02315. eCollection 2018. Front Immunol. 2018. PMID: 30349542 Free PMC article. Review.
-
Transcriptional signatures measured in whole blood correlate with protection against tuberculosis in inbred and outbred mice.PLoS One. 2023 Aug 3;18(8):e0289358. doi: 10.1371/journal.pone.0289358. eCollection 2023. PLoS One. 2023. PMID: 37535648 Free PMC article.
References
-
- WHO. 2014. Global tuberculosis report 2014. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf?....
-
- Brennan MJ, Thole J. 2012. Tuberculosis vaccines: a strategic blueprint for the next decade. Tuberculosis (Edinb) 92(Suppl 1):S6–S13. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

