Replication and Inhibitors of Enteroviruses and Parechoviruses
- PMID: 26266417
- PMCID: PMC4576193
- DOI: 10.3390/v7082832
Replication and Inhibitors of Enteroviruses and Parechoviruses
Abstract
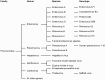
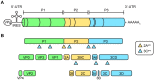
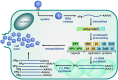
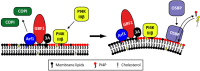
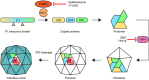
The Enterovirus (EV) and Parechovirus genera of the picornavirus family include many important human pathogens, including poliovirus, rhinovirus, EV-A71, EV-D68, and human parechoviruses (HPeV). They cause a wide variety of diseases, ranging from a simple common cold to life-threatening diseases such as encephalitis and myocarditis. At the moment, no antiviral therapy is available against these viruses and it is not feasible to develop vaccines against all EVs and HPeVs due to the great number of serotypes. Therefore, a lot of effort is being invested in the development of antiviral drugs. Both viral proteins and host proteins essential for virus replication can be used as targets for virus inhibitors. As such, a good understanding of the complex process of virus replication is pivotal in the design of antiviral strategies goes hand in hand with a good understanding of the complex process of virus replication. In this review, we will give an overview of the current state of knowledge of EV and HPeV replication and how this can be inhibited by small-molecule inhibitors.
Keywords: antiviral; enterovirus; human parechovirus; inhibitor; replication; small molecules.
Figures

Similar articles
-
Antiviral effects of selected nucleoside analogues against human parechoviruses A1 and A3.Antiviral Res. 2019 Feb;162:51-53. doi: 10.1016/j.antiviral.2018.12.009. Epub 2018 Dec 12. Antiviral Res. 2019. PMID: 30550798
-
Combating enterovirus replication: state-of-the-art on antiviral research.Biochem Pharmacol. 2012 Jan 15;83(2):185-92. doi: 10.1016/j.bcp.2011.08.016. Epub 2011 Aug 26. Biochem Pharmacol. 2012. PMID: 21889497 Review.
-
Doxycycline inhibits neurotropic enterovirus proliferation in vitro.Virus Res. 2024 Jul;345:199388. doi: 10.1016/j.virusres.2024.199388. Epub 2024 May 16. Virus Res. 2024. PMID: 38714218 Free PMC article.
-
Antiviral Peptides Targeting the Helicase Activity of Enterovirus Nonstructural Protein 2C.J Virol. 2021 May 24;95(12):e02324-20. doi: 10.1128/JVI.02324-20. Print 2021 May 24. J Virol. 2021. PMID: 33789997 Free PMC article.
-
Development of antiviral agents for enteroviruses.J Antimicrob Chemother. 2008 Dec;62(6):1169-73. doi: 10.1093/jac/dkn424. Epub 2008 Oct 18. J Antimicrob Chemother. 2008. PMID: 18931391 Review.
Cited by
-
Mutations in VP1 and 3A proteins improve binding and replication of rhinovirus C15 in HeLa-E8 cells.Virology. 2016 Dec;499:350-360. doi: 10.1016/j.virol.2016.09.025. Epub 2016 Oct 13. Virology. 2016. PMID: 27743961 Free PMC article.
-
In Vitro Assessment of Combinations of Enterovirus Inhibitors against Enterovirus 71.Antimicrob Agents Chemother. 2016 Aug 22;60(9):5357-67. doi: 10.1128/AAC.01073-16. Print 2016 Sep. Antimicrob Agents Chemother. 2016. PMID: 27353263 Free PMC article.
-
Enterovirus and parechovirus infection in children: a brief overview.Eur J Pediatr. 2016 Aug;175(8):1023-9. doi: 10.1007/s00431-016-2725-7. Epub 2016 May 7. Eur J Pediatr. 2016. PMID: 27156106 Free PMC article. Review.
-
Antiviral Agents From Fungi: Diversity, Mechanisms and Potential Applications.Front Microbiol. 2018 Oct 2;9:2325. doi: 10.3389/fmicb.2018.02325. eCollection 2018. Front Microbiol. 2018. PMID: 30333807 Free PMC article. Review.
-
CRISPR-Cas9-Based Technology for Studying Enteric Virus Infection.Front Genome Ed. 2022 Jun 8;4:888878. doi: 10.3389/fgeed.2022.888878. eCollection 2022. Front Genome Ed. 2022. PMID: 35755450 Free PMC article. Review.
References
-
- Knowles N.J., Hovi T., Hyypiä T., King A.M., Lindberg A.M., Pallansch M.A., Palmenberg A.C., Simmonds P., Skern T., Stanway G., et al. Picornaviridae. In: King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J., editors. Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier; San Diego, CA, USA: 2012. pp. 855–880.
-
- Centers for Disease Control and Prevention . Poliomyelitis. In: Atkinson W., Hamborsky J., Wolfe S., editors. Epidemiology and Prevention of Vaccine-Preventable Diseases. Volume 12. Public Health Foundation; Washington DC: 2012. pp. 249–261.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

