Pharmacological antagonism of interleukin-8 receptor CXCR2 inhibits inflammatory reactivity and is neuroprotective in an animal model of Alzheimer's disease
- PMID: 26255110
- PMCID: PMC4529987
- DOI: 10.1186/s12974-015-0339-z
Pharmacological antagonism of interleukin-8 receptor CXCR2 inhibits inflammatory reactivity and is neuroprotective in an animal model of Alzheimer's disease
Abstract
Background: The chemokine interleukin-8 (IL-8) and its receptor CXCR2 contribute to chemotactic responses in Alzheimer's disease (AD); however, properties of the ligand and receptor have not been characterized in animal models of disease. The primary aim of our study was to examine effects of pharmacological antagonism of CXCR2 as a strategy to inhibit receptor-mediated inflammatory reactivity and enhance neuronal viability in animals receiving intrahippocampal injection of amyloid-beta (Aβ1-42).
Methods: In vivo studies used an animal model of Alzheimer's disease incorporating injection of full-length Aβ1-42 into rat hippocampus. Immunohistochemical staining of rat brain was used to measure microgliosis, astrogliosis, neuronal viability, and oxidative stress. Western blot and Reverse Transcription PCR (RT-PCR) were used to determine levels of CXCR2 in animal tissue with the latter also used to determine expression of pro-inflammatory mediators. Immunostaining of human AD and non-demented (ND) tissue was also undertaken.
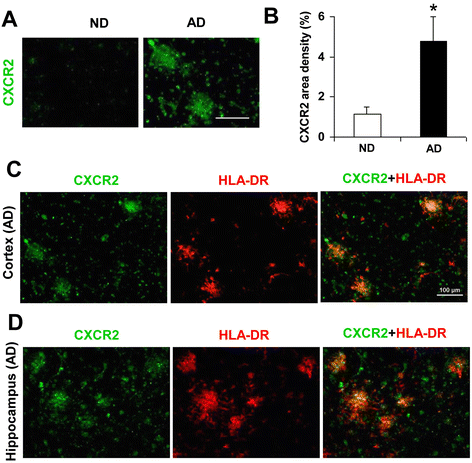
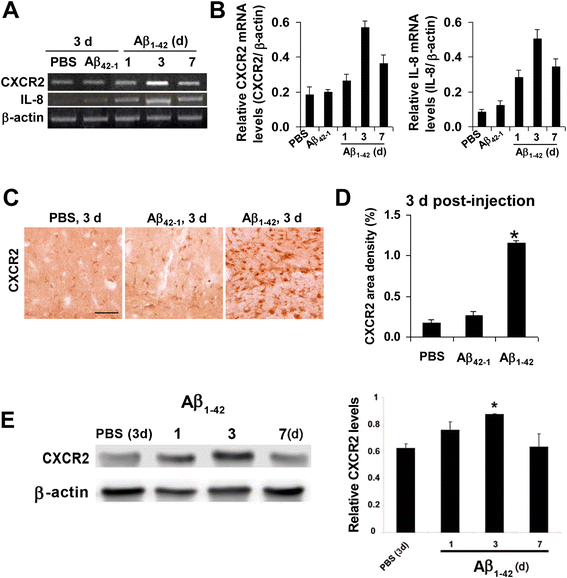
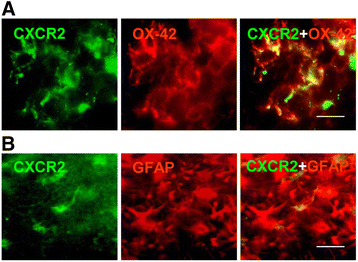
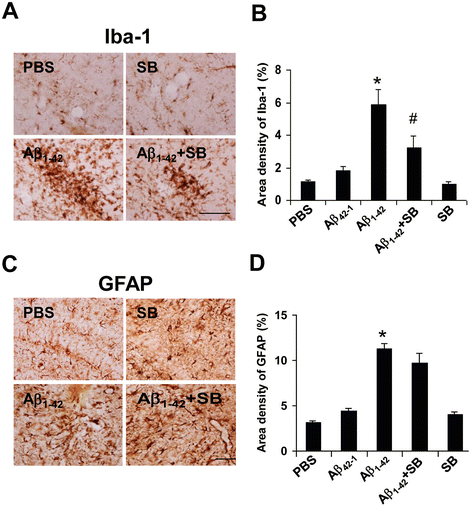
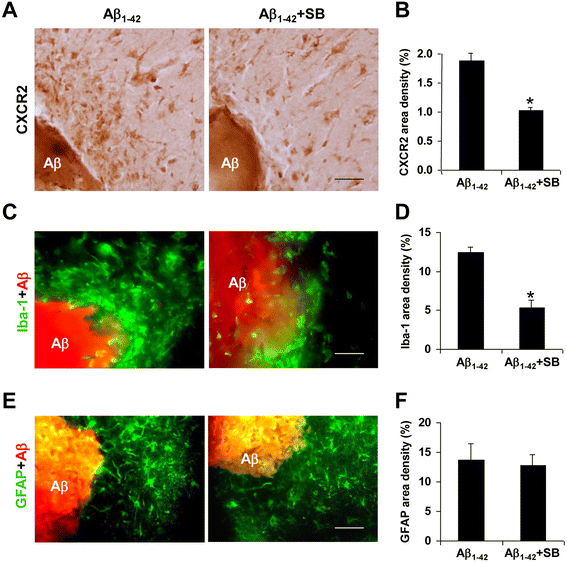
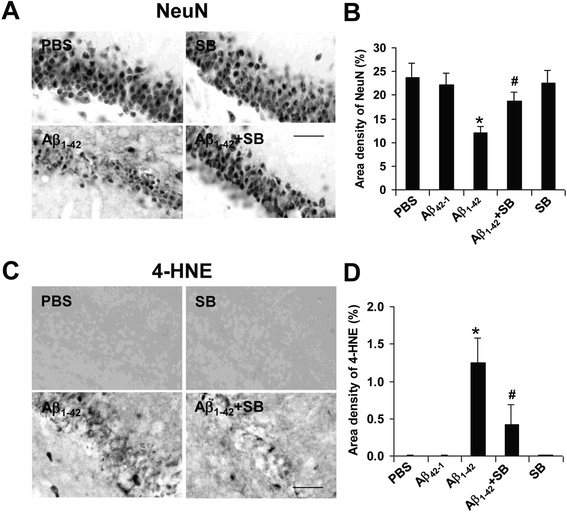
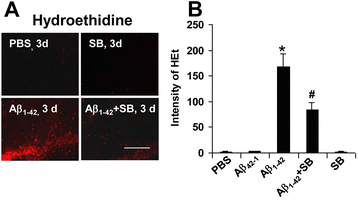
Results: We initially determined that in the human brain, AD relative to ND tissue exhibited marked increases in expression of CXCR2 with cell-specific receptor expression prominent in microglia. In Aβ1-42-injected rat brain, CXCR2 and IL-8 showed time-dependent increases in expression, concomitant with enhanced gliosis, relative to controls phosphate-buffered saline (PBS) or reverse peptide Aβ42-1 injection. Administration of the competitive CXCR2 antagonist SB332235 to peptide-injected rats significantly reduced expression of CXCR2 and microgliosis, with astrogliosis unchanged. Double staining studies demonstrated localization of CXCR2 and microglial immunoreactivity nearby deposits of Aβ1-42 with SB332235 effective in inhibiting receptor expression and microgliosis. The numbers of neurons in granule cell layer (GCL) were reduced in rats receiving Aβ1-42, compared with PBS, with administration of SB332235 to peptide-injected animals conferring neuroprotection. Oxidative stress was indicated in the animal model since both 4-hydroxynonenal (4-HNE) and hydroethidine (HEt) were markedly elevated in Aβ1-42 vs. PBS-injected rat brain and diminished with SB332235 treatment.
Conclusion: Overall, the findings suggest critical roles for CXCR2-dependent inflammatory responses in an AD animal model with pharmacological modulation of the receptor effective in inhibiting inflammatory reactivity and conferring neuroprotection against oxidative damage.
Figures
Similar articles
-
Neural progenitor cells attenuate inflammatory reactivity and neuronal loss in an animal model of inflamed AD brain.J Neuroinflammation. 2009 Dec 23;6:39. doi: 10.1186/1742-2094-6-39. J Neuroinflammation. 2009. PMID: 20030829 Free PMC article.
-
Protection of TGF-β1 against neuroinflammation and neurodegeneration in Aβ1-42-induced Alzheimer's disease model rats.PLoS One. 2015 Feb 6;10(2):e0116549. doi: 10.1371/journal.pone.0116549. eCollection 2015. PLoS One. 2015. PMID: 25658940 Free PMC article.
-
A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer's disease brain.J Cell Mol Med. 2009 Sep;13(9A):2911-25. doi: 10.1111/j.1582-4934.2008.00434.x. Epub 2008 Jul 24. J Cell Mol Med. 2009. PMID: 18657226 Free PMC article.
-
Relevance of abeta1-42 intrahippocampal injection as an animal model of inflamed Alzheimer's disease brain.Curr Alzheimer Res. 2008 Oct;5(5):475-80. doi: 10.2174/156720508785908874. Curr Alzheimer Res. 2008. PMID: 18855589 Review.
-
Correlated inflammatory responses and neurodegeneration in peptide-injected animal models of Alzheimer's disease.Biomed Res Int. 2014;2014:923670. doi: 10.1155/2014/923670. Epub 2014 Apr 13. Biomed Res Int. 2014. PMID: 24822221 Free PMC article. Review.
Cited by
-
Identification of Genes Responding to Iron or Choline Treatment for Early-Life Iron Deficiency in the Male Rat Hippocampal Transcriptomes.J Nutr. 2024 Apr;154(4):1141-1152. doi: 10.1016/j.tjnut.2024.02.021. Epub 2024 Feb 24. J Nutr. 2024. PMID: 38408730
-
Pathophysiology and Therapeutic Perspectives of Oxidative Stress and Neurodegenerative Diseases: A Narrative Review.Adv Ther. 2020 Jan;37(1):113-139. doi: 10.1007/s12325-019-01148-5. Epub 2019 Nov 28. Adv Ther. 2020. PMID: 31782132 Free PMC article.
-
Comparisons of Serum Interleukin-8 Levels in Major Depressive Patients With Drug-Free Versus SSRIs Versus Healthy Controls.Front Psychiatry. 2022 Apr 14;13:858675. doi: 10.3389/fpsyt.2022.858675. eCollection 2022. Front Psychiatry. 2022. PMID: 35492731 Free PMC article.
-
Roles of Microglial and Monocyte Chemokines and Their Receptors in Regulating Alzheimer's Disease-Associated Amyloid-β and Tau Pathologies.Front Neurol. 2018 Aug 14;9:549. doi: 10.3389/fneur.2018.00549. eCollection 2018. Front Neurol. 2018. PMID: 30158892 Free PMC article. Review.
-
Association of gut microbiota with sort-chain fatty acids and inflammatory cytokines in diabetic patients with cognitive impairment: A cross-sectional, non-controlled study.Front Nutr. 2022 Jul 22;9:930626. doi: 10.3389/fnut.2022.930626. eCollection 2022. Front Nutr. 2022. PMID: 35938126 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

