A common glycan structure on immunoglobulin G for enhancement of effector functions
- PMID: 26253764
- PMCID: PMC4553773
- DOI: 10.1073/pnas.1513456112
A common glycan structure on immunoglobulin G for enhancement of effector functions
Abstract
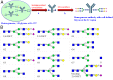
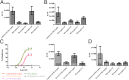
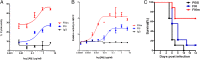
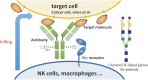
Antibodies have been developed as therapeutic agents for the treatment of cancer, infection, and inflammation. In addition to binding activity toward the target, antibodies also exhibit effector-mediated activities through the interaction of the Fc glycan and the Fc receptors on immune cells. To identify the optimal glycan structures for individual antibodies with desired activity, we have developed an effective method to modify the Fc-glycan structures to a homogeneous glycoform. In this study, it was found that the biantennary N-glycan structure with two terminal alpha-2,6-linked sialic acids is a common and optimized structure for the enhancement of antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity, and antiinflammatory activities.
Keywords: Fc glycosylation; endoglycosidase; glycoengineered antibodies; homogeneous antibodies; sugar oxazoline.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Crystal Structure of a Homogeneous IgG-Fc Glycoform with the N-Glycan Designed to Maximize the Antibody Dependent Cellular Cytotoxicity.ACS Chem Biol. 2017 May 19;12(5):1335-1345. doi: 10.1021/acschembio.7b00140. Epub 2017 Mar 31. ACS Chem Biol. 2017. PMID: 28318221
-
Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity.J Clin Invest. 2015 Nov 2;125(11):4160-70. doi: 10.1172/JCI82695. Epub 2015 Oct 5. J Clin Invest. 2015. PMID: 26436649 Free PMC article. Clinical Trial.
-
Effects of terminal galactose residues in mannose α1-6 arm of Fc-glycan on the effector functions of therapeutic monoclonal antibodies.MAbs. 2019 Jul;11(5):826-836. doi: 10.1080/19420862.2019.1608143. Epub 2019 May 8. MAbs. 2019. PMID: 30990348 Free PMC article.
-
Terminal sugars of Fc glycans influence antibody effector functions of IgGs.Curr Opin Immunol. 2008 Aug;20(4):471-8. doi: 10.1016/j.coi.2008.06.007. Epub 2008 Jul 17. Curr Opin Immunol. 2008. PMID: 18606225 Review.
-
Impact of differential glycosylation on IgG activity.Adv Exp Med Biol. 2011;780:113-24. doi: 10.1007/978-1-4419-5632-3_10. Adv Exp Med Biol. 2011. PMID: 21842369 Review.
Cited by
-
Glycosynthase Mutants of Endoglycosidase S2 Show Potent Transglycosylation Activity and Remarkably Relaxed Substrate Specificity for Antibody Glycosylation Remodeling.J Biol Chem. 2016 Aug 5;291(32):16508-18. doi: 10.1074/jbc.M116.738765. Epub 2016 Jun 10. J Biol Chem. 2016. PMID: 27288408 Free PMC article.
-
Increased expression of SSEA-4 on TKI-resistant non-small cell lung cancer with EGFR-T790M mutation.Proc Natl Acad Sci U S A. 2024 Jan 30;121(5):e2313397121. doi: 10.1073/pnas.2313397121. Epub 2024 Jan 22. Proc Natl Acad Sci U S A. 2024. PMID: 38252815 Free PMC article.
-
Chemoenzymatic Methods for the Synthesis of Glycoproteins.Chem Rev. 2018 Sep 12;118(17):8359-8413. doi: 10.1021/acs.chemrev.8b00238. Epub 2018 Aug 24. Chem Rev. 2018. PMID: 30141327 Free PMC article. Review.
-
Glycosylation-on-a-Chip: A Flow-Based Microfluidic System for Cell-Free Glycoprotein Biosynthesis.Front Mol Biosci. 2021 Dec 23;8:782905. doi: 10.3389/fmolb.2021.782905. eCollection 2021. Front Mol Biosci. 2021. PMID: 35004852 Free PMC article.
-
O-Glycosylation Changes in Serum Immunoglobulin G Are Associated with Inflammation Development in Advanced Endometriosis.Int J Mol Sci. 2022 Jul 22;23(15):8087. doi: 10.3390/ijms23158087. Int J Mol Sci. 2022. PMID: 35897676 Free PMC article.
References
-
- Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–497. - PubMed
-
- McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: Filamentous phage displaying antibody variable domains. Nature. 1990;348(6301):552–554. - PubMed
-
- Shields RL, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277(30):26733–26740. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

