Porcine reproductive and respiratory syndrome virus 3C protease cleaves the mitochondrial antiviral signalling complex to antagonize IFN-β expression
- PMID: 26253126
- PMCID: PMC5410108
- DOI: 10.1099/jgv.0.000257
Porcine reproductive and respiratory syndrome virus 3C protease cleaves the mitochondrial antiviral signalling complex to antagonize IFN-β expression
Abstract
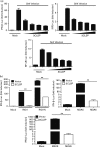
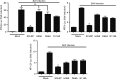
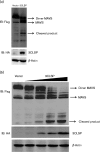
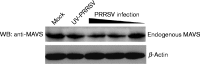
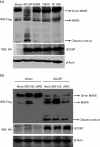
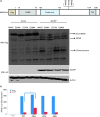
Porcine reproductive and respiratory syndrome, a highly infectious disease caused by porcine reproductive and respiratory syndrome virus (PRRSV), has developed various strategies to evade the host innate immune response, including the suppression of type I IFN activation. The mitochondrial antiviral signalling protein (MAVS) is an important bridging adaptor of retinoic acid-inducible gene I/melanoma differentiation-associated protein 5 signalling pathways. Here, we demonstrated that the 3C-like protease (3CLSP) of PRRSV prevented the induction of IFN-β by cleaving MAVS in a proteasome- and caspase-independent manner. Moreover, this cleavage ability was dependent on the protease activity of 3CLSP. Mutations specifically disrupting the cysteine protease activity of 3CLSP eliminated MAVS cleavage and the inhibition of IFN induction. Subsequently, we determined that 3CLSP cleaved MAVS at Glu268. Remarkably, a MAVS point mutation at Glu268 rendered MAVS resistant to 3CLSP cleavage. These results reveal a novel PRRSV mechanism to escape host immunity by directly cleaving MAVS.
Figures

Similar articles
-
Seneca Valley Virus Suppresses Host Type I Interferon Production by Targeting Adaptor Proteins MAVS, TRIF, and TANK for Cleavage.J Virol. 2017 Jul 27;91(16):e00823-17. doi: 10.1128/JVI.00823-17. Print 2017 Aug 15. J Virol. 2017. PMID: 28566380 Free PMC article.
-
USP8 suppresses porcine reproductive and respiratory syndrome virus replication by positively regulating MAVS mediated Ⅰ-IFN signaling.Vet Microbiol. 2024 Nov;298:110286. doi: 10.1016/j.vetmic.2024.110286. Epub 2024 Oct 28. Vet Microbiol. 2024. PMID: 39509836
-
Arterivirus nsp4 Antagonizes Interferon Beta Production by Proteolytically Cleaving NEMO at Multiple Sites.J Virol. 2019 May 29;93(12):e00385-19. doi: 10.1128/JVI.00385-19. Print 2019 Jun 15. J Virol. 2019. PMID: 30944180 Free PMC article.
-
Mechanisms of suppression of interferon production by porcine reproductive and respiratory syndrome virus.Acta Virol. 2012;56(1):3-9. doi: 10.4149/av_2012_01_3. Acta Virol. 2012. PMID: 22404603 Review.
-
Modulation of host cell responses and evasion strategies for porcine reproductive and respiratory syndrome virus.Virus Res. 2010 Dec;154(1-2):48-60. doi: 10.1016/j.virusres.2010.07.019. Epub 2010 Jul 23. Virus Res. 2010. PMID: 20655963 Free PMC article. Review.
Cited by
-
Current Status of Vaccines for Porcine Reproductive and Respiratory Syndrome: Interferon Response, Immunological Overview, and Future Prospects.Vaccines (Basel). 2024 Jun 1;12(6):606. doi: 10.3390/vaccines12060606. Vaccines (Basel). 2024. PMID: 38932335 Free PMC article. Review.
-
Innate immune evasion strategies of DNA and RNA viruses.Curr Opin Microbiol. 2016 Aug;32:113-119. doi: 10.1016/j.mib.2016.05.015. Epub 2016 Jun 8. Curr Opin Microbiol. 2016. PMID: 27288760 Free PMC article. Review.
-
Tembusu Virus Nonstructural Protein 2B Antagonizes Type I Interferon Production by Targeting MAVS for Degradation.J Virol. 2022 Jul 27;96(14):e0081622. doi: 10.1128/jvi.00816-22. Epub 2022 Jul 11. J Virol. 2022. PMID: 35867574 Free PMC article.
-
Immune Ecosystem of Virus-Infected Host Tissues.Int J Mol Sci. 2018 May 6;19(5):1379. doi: 10.3390/ijms19051379. Int J Mol Sci. 2018. PMID: 29734779 Free PMC article. Review.
-
Ursonic acid from medicinal herbs inhibits PRRSV replication through activation of the innate immune response by targeting the phosphatase PTPN1.Vet Res. 2024 May 23;55(1):67. doi: 10.1186/s13567-024-01316-8. Vet Res. 2024. PMID: 38783392 Free PMC article.
References
-
- Beura L.K., Sarkar S.N., Kwon B., Subramaniam S., Jones C., Pattnaik A.K., Osorio F.A. (2010). Porcine reproductive and respiratory syndrome virus nonstructural protein 1β modulates host innate immune response by antagonizing IRF3 activation J Virol 841574–158410.1128/JVI.01326-09. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

