Efficacy of topical tenofovir against transmission of a tenofovir-resistant SHIV in macaques
- PMID: 26253002
- PMCID: PMC4528693
- DOI: 10.1186/s12977-015-0195-z
Efficacy of topical tenofovir against transmission of a tenofovir-resistant SHIV in macaques
Abstract
Background: Topically delivered tenofovir (TFV) from intravaginal rings, tablets, or gels is being evaluated for HIV prevention. We previously demonstrated that TFV delivered vaginally by gel protected macaques from vaginal infection with SHIV. Here we investigated efficacy of the TFV gel against vaginal transmission of a TFV-resistant SHIV containing the K65R mutation (SHIV162P3K65R) and its relationship to drug levels in vaginal tissues.
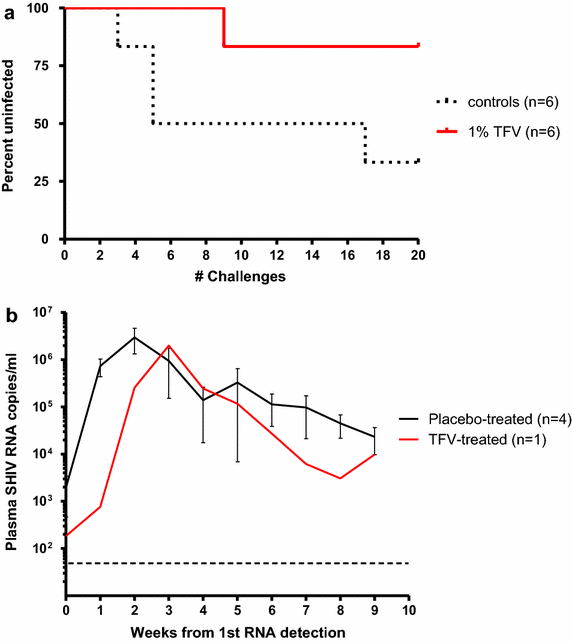
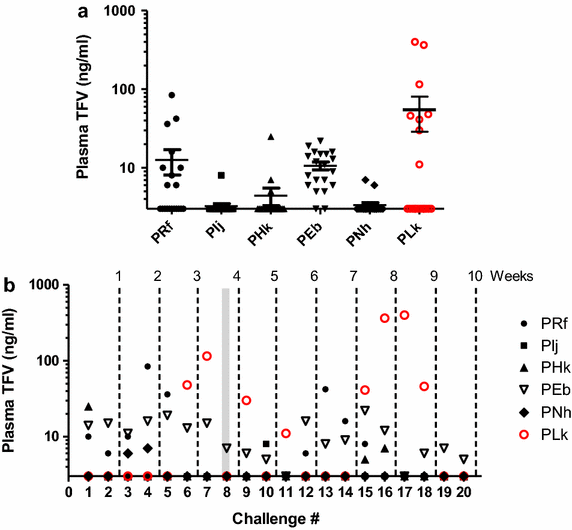
Results: SHIV162P3K65R shows approximately a 5-fold reduction in susceptibility to TFV compared to wild-type SHIV. Efficacy was evaluated in pig-tailed macaques exposed vaginally twice-weekly (up to 10 weeks) to SHIV162P3K65R 30 min after receiving placebo (n = 6) or 1% TFV (n = 6) gel. Four of the six controls were infected after a median of 5 exposures. In contrast, five of six macaques that received TFV gel remained uninfected after 20 vaginal SHIV162P3K65R exposures, resulting in an estimated efficacy of 75%. The mean intracellular TFV-diphosphate (TFV-DP) concentrations in vaginal lymphocytes 4 h after a single gel dose were found to be high (1,631 fmol/10(6) cells, range 492-3,847) and within the in vitro IC75 range (1,206 fmol/10(6) cells) for SHIV162P3K65R.
Conclusion: Both the modest resistance conferred by K65R and the high TFV-DP exposure in vaginal lymphocytes, likely explain the observed protection. The findings in this model do not predict complete loss of protection by topical TFV against vaginal exposure to HIV-1K65R viruses and provide a tissue drug target for high efficacy. These data will facilitate the development of TFV delivery platforms that have high activity on both wild-type and TFV-resistant viruses.
Figures
Similar articles
-
Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue.J Virol. 2012 Jan;86(2):718-25. doi: 10.1128/JVI.05842-11. Epub 2011 Nov 9. J Virol. 2012. PMID: 22072766 Free PMC article.
-
Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine.J Virol. 2009 Oct;83(20):10358-65. doi: 10.1128/JVI.01073-09. Epub 2009 Aug 5. J Virol. 2009. PMID: 19656878 Free PMC article.
-
Topical tenofovir protects against vaginal simian HIV infection in macaques coinfected with Chlamydia trachomatis and Trichomonas vaginalis.AIDS. 2017 Mar 27;31(6):745-752. doi: 10.1097/QAD.0000000000001389. AIDS. 2017. PMID: 28060011 Free PMC article.
-
The Vaginal Microbiome and its Potential to Impact Efficacy of HIV Pre-exposure Prophylaxis for Women.Curr HIV/AIDS Rep. 2017 Oct;14(5):153-160. doi: 10.1007/s11904-017-0362-z. Curr HIV/AIDS Rep. 2017. PMID: 28812207 Free PMC article. Review.
-
Animal models of antiretroviral prophylaxis for HIV prevention.Curr Opin HIV AIDS. 2012 Nov;7(6):505-13. doi: 10.1097/COH.0b013e328358e484. Curr Opin HIV AIDS. 2012. PMID: 22964889 Review.
Cited by
-
Preclinical and Early Clinical Development of Tenofovir Alafenamide/Elvitegravir Topical Inserts for Effective On-Demand Vaginal and Rectal HIV Prevention.Pharmaceutics. 2024 Mar 1;16(3):348. doi: 10.3390/pharmaceutics16030348. Pharmaceutics. 2024. PMID: 38543242 Free PMC article.
-
A phase I study to assess safety, pharmacokinetics, and pharmacodynamics of a vaginal insert containing tenofovir alafenamide and elvitegravir.Front Cell Infect Microbiol. 2023 Apr 19;13:1130101. doi: 10.3389/fcimb.2023.1130101. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37153145 Free PMC article. Clinical Trial.
-
Translational Models to Predict Target Concentrations for Pre-Exposure Prophylaxis in Women.AIDS Res Hum Retroviruses. 2022 Dec;38(12):909-923. doi: 10.1089/AID.2022.0057. Epub 2022 Oct 25. AIDS Res Hum Retroviruses. 2022. PMID: 36097755 Free PMC article. Review.
-
Nonhuman primate models for the evaluation of HIV-1 preventive vaccine strategies: model parameter considerations and consequences.Curr Opin HIV AIDS. 2016 Nov;11(6):546-554. doi: 10.1097/COH.0000000000000311. Curr Opin HIV AIDS. 2016. PMID: 27559710 Free PMC article. Review.
-
Recent Insights into the HIV/AIDS Pandemic.Microb Cell. 2016 Sep 5;3(9):451-475. doi: 10.15698/mic2016.09.529. Microb Cell. 2016. PMID: 28357381 Free PMC article. Review.
References
-
- UNAIDS . TJUNPoHA: UNAIDS/WHO. Report on the global HIV/AIDS epidemic 2013. Geneva: UNAIDS; 2013.
-
- Administration (2013) FaD: FDA approves first medication to reduce HIV risk. 16 July 2012
-
- Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. - DOI - PMC - PubMed
-
- Rees H, Delany-Moretlwe S, Baron D, Lombard C, Gray G, Myer L, Panchia R, Schwartz J, Doncel G. FACTS 001 Phase III trial of pericoital tenofovir 1% gel for HIV prevention in women [abstract 26LB]. Program and abstracts of the 2015 Conference on Retroviruses and Opportunistic Infections (CROI) Seattle: CROI; 2015.
-
- Moss JA, Srinivasan P, Smith TJ, Butkyavichene I, Lopez G, Brooks AA, Martin A, Dinh CT, Smith JM, Baum MM. Pharmacokinetics and preliminary safety study of pod-intravaginal rings delivering antiretroviral combinations for HIV prophylaxis in a macaque model. Antimicrob Agents Chemother. 2014;58:5125–5135. doi: 10.1128/AAC.02871-14. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

