Structure of BbKI, a disulfide-free plasma kallikrein inhibitor
- PMID: 26249699
- PMCID: PMC4528941
- DOI: 10.1107/S2053230X15011127
Structure of BbKI, a disulfide-free plasma kallikrein inhibitor
Abstract
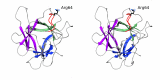
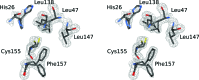
A serine protease inhibitor from Bauhinia bauhinioides (BbKI) belongs to the Kunitz family of plant inhibitors, which are common in plant seeds. BbKI does not contain any disulfides, unlike most other members of this family. It is a potent inhibitor of plasma kallikrein, in addition to other serine proteases, and thus exhibits antithrombotic activity. A high-resolution crystal structure of recombinantly expressed BbKI was determined (at 1.4 Å resolution) and was compared with the structures of other members of the family. Modeling of a complex of BbKI with plasma kallikrein indicates that changes in the local structure of the reactive loop that includes the specificity-determining Arg64 are necessary in order to explain the tight binding. An R64A mutant of BbKI was found to be a weaker inhibitor of plasma kallikrein, but was much more potent against plasmin, suggesting that this mutant may be useful for preventing the breakup of fibrin and maintaining clot stability, thus preventing excessive bleeding.
Keywords: Kunitz inhibitor; crystal structure; kallikrein; β-trefoil.
Figures

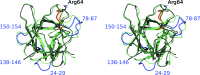
Similar articles
-
Crystal structures of the complex of a kallikrein inhibitor from Bauhinia bauhinioides with trypsin and modeling of kallikrein complexes.Acta Crystallogr D Struct Biol. 2019 Jan 1;75(Pt 1):56-69. doi: 10.1107/S2059798318016492. Epub 2019 Jan 7. Acta Crystallogr D Struct Biol. 2019. PMID: 30644845 Free PMC article.
-
Structural studies of complexes of kallikrein 4 with wild-type and mutated forms of the Kunitz-type inhibitor BbKI.Acta Crystallogr D Struct Biol. 2021 Aug 1;77(Pt 8):1084-1098. doi: 10.1107/S2059798321006483. Epub 2021 Jul 29. Acta Crystallogr D Struct Biol. 2021. PMID: 34342281 Free PMC article.
-
Kunitz-type Bauhinia bauhinioides inhibitors devoid of disulfide bridges: isolation of the cDNAs, heterologous expression and structural studies.Biol Chem. 2005 Jun;386(6):561-8. doi: 10.1515/BC.2005.066. Biol Chem. 2005. PMID: 16006243
-
Action of plant proteinase inhibitors on enzymes of physiopathological importance.An Acad Bras Cienc. 2009 Sep;81(3):615-21. doi: 10.1590/s0001-37652009000300023. An Acad Bras Cienc. 2009. PMID: 19722028 Review.
-
Analysis of Kunitz inhibitors from plants for comprehensive structural and functional insights.Int J Biol Macromol. 2018 Jul 1;113:933-943. doi: 10.1016/j.ijbiomac.2018.02.148. Epub 2018 Feb 27. Int J Biol Macromol. 2018. PMID: 29499268 Review.
Cited by
-
The Plant-Derived Bauhinia bauhinioides Kallikrein Proteinase Inhibitor (rBbKI) Attenuates Elastase-Induced Emphysema in Mice.Mediators Inflamm. 2016;2016:5346574. doi: 10.1155/2016/5346574. Epub 2016 Aug 1. Mediators Inflamm. 2016. PMID: 27528793 Free PMC article.
-
MnASI1 Mediates Resistance to Botrytis cinerea in Mulberry (Morus notabilis).Int J Mol Sci. 2022 Nov 2;23(21):13372. doi: 10.3390/ijms232113372. Int J Mol Sci. 2022. PMID: 36362160 Free PMC article.
-
Crystal structures of the complex of a kallikrein inhibitor from Bauhinia bauhinioides with trypsin and modeling of kallikrein complexes.Acta Crystallogr D Struct Biol. 2019 Jan 1;75(Pt 1):56-69. doi: 10.1107/S2059798318016492. Epub 2019 Jan 7. Acta Crystallogr D Struct Biol. 2019. PMID: 30644845 Free PMC article.
-
Structural studies of complexes of kallikrein 4 with wild-type and mutated forms of the Kunitz-type inhibitor BbKI.Acta Crystallogr D Struct Biol. 2021 Aug 1;77(Pt 8):1084-1098. doi: 10.1107/S2059798321006483. Epub 2021 Jul 29. Acta Crystallogr D Struct Biol. 2021. PMID: 34342281 Free PMC article.
-
Plant-Derived Compounds and Extracts as Modulators of Plasmin Activity-A Review.Molecules. 2023 Feb 9;28(4):1677. doi: 10.3390/molecules28041677. Molecules. 2023. PMID: 36838662 Free PMC article. Review.
References
-
- Araújo, A. P. U., Hansen, D., Vieira, D. F., Oliveira, C., Santana, L. A., Beltramini, L. M., Sampaio, C. A. M., Sampaio, M. U. & Oliva, M. L. V. (2005). Biol. Chem. 386, 561–568. - PubMed
-
- Batista, I. F. C., Oliva, M. L. V., Araujo, M. S., Sampaio, M. U., Richardson, M., Fritz, H. & Sampaio, C. A. M. (1996). Phytochemistry, 41, 1017–1022. - PubMed
-
- Bhattacharjee, N., Banerjee, S. & Dutta, S. K. (2014). Protein Expr. Purif. 96, 26–31. - PubMed
-
- Birk, Y. (2003). Plant Protease Inhibitors. Berlin: Springer Verlag.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

