An IPTG Inducible Conditional Expression System for Mycobacteria
- PMID: 26247874
- PMCID: PMC4527713
- DOI: 10.1371/journal.pone.0134562
An IPTG Inducible Conditional Expression System for Mycobacteria
Abstract
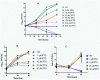
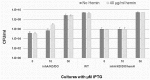
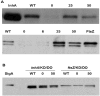
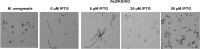
Conditional expression strains serve as a valuable tool to study the essentiality and to establish the vulnerability of a target under investigation in a drug discovery program. While essentiality implies an absolute requirement of a target function, vulnerability provides valuable information on the extent to which a target function needs to be depleted to achieve bacterial growth inhibition followed by cell death. The critical feature of an ideal conditional expression system is its ability to tightly regulate gene expression to achieve the full spectrum spanning from a high level of expression in order to support growth and near zero level of expression to mimic conditions of gene knockout. A number of bacterial conditional expression systems have been reported for use in mycobacteria. The utility of an isopropylthiogalactoside (IPTG) inducible system in mycobacteria has been reported for protein overexpression and anti-sense gene expression from a replicating multi-copy plasmid. Herein, we report the development of a versatile set of non-replicating IPTG inducible vectors for mycobacteria which can be used for generation of conditional expression strains through homologous recombination. The role of a single lac operator versus a double lac operator to regulate gene expression was evaluated by monitoring the expression levels of β-galactosidase in Mycobacterium smegmatis. These studies indicated a significant level of leaky expression from the vector with a single lac operator but none from the vector with double lac operator. The significance of the double lac operator vector for target validation was established by monitoring the growth kinetics of an inhA, a rpoB and a ftsZ conditional expression strain grown in the presence of different concentrations of IPTG. The utility of this inducible system in identifying target specific inhibitors was established by screening a focussed library of small molecules using an inhA and a rpoB conditional expression strain.
Conflict of interest statement
Figures




Similar articles
-
Vectors for regulated gene expression in the radioresistant bacterium Deinococcus radiodurans.Gene. 2004 Jul 7;336(1):25-35. doi: 10.1016/j.gene.2004.04.006. Gene. 2004. PMID: 15225873
-
Regulation of the Escherichia coli lac operon expressed in human cells.Biochim Biophys Acta. 1992 Feb 28;1130(1):68-74. doi: 10.1016/0167-4781(92)90463-a. Biochim Biophys Acta. 1992. PMID: 1311956
-
Development of inducer-free expression plasmids based on IPTG-inducible promoters for Bacillus subtilis.Microb Cell Fact. 2017 Jul 25;16(1):130. doi: 10.1186/s12934-017-0747-0. Microb Cell Fact. 2017. PMID: 28743271 Free PMC article.
-
Delineating bacteriostatic and bactericidal targets in mycobacteria using IPTG inducible antisense expression.PLoS One. 2009 Jun 15;4(6):e5923. doi: 10.1371/journal.pone.0005923. PLoS One. 2009. PMID: 19526063 Free PMC article.
-
Controlling the expression of rhizobial genes during nodule development with elements and an inducer of the lac operon.Mol Plant Microbe Interact. 2011 Apr;24(4):478-86. doi: 10.1094/MPMI-07-10-0155. Mol Plant Microbe Interact. 2011. PMID: 21375387
Cited by
-
An Adapted GeneSwitch Toolkit for Comparable Cellular and Animal Models: A Proof of Concept in Modeling Charcot-Marie-Tooth Neuropathy.Int J Mol Sci. 2023 Nov 9;24(22):16138. doi: 10.3390/ijms242216138. Int J Mol Sci. 2023. PMID: 38003325 Free PMC article.
-
Essentiality Assessment of Cysteinyl and Lysyl-tRNA Synthetases of Mycobacterium smegmatis.PLoS One. 2016 Jan 21;11(1):e0147188. doi: 10.1371/journal.pone.0147188. eCollection 2016. PLoS One. 2016. PMID: 26794499 Free PMC article.
-
A uniform cloning platform for mycobacterial genetics and protein production.Sci Rep. 2018 Jun 22;8(1):9539. doi: 10.1038/s41598-018-27687-5. Sci Rep. 2018. PMID: 29934571 Free PMC article.
-
Plasmid to generate Mycobacteria mutants.AMB Express. 2018 Feb 1;8(1):13. doi: 10.1186/s13568-018-0537-z. AMB Express. 2018. PMID: 29392444 Free PMC article.
References
-
- Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL (2007) Drugs for Bad Bugs: Confronting the Challenges of Antibacterial Discovery. Nature Rev Drug Discovery 6: 29–40. - PubMed
-
- Chan PF, Macarron R, Payne DJ, Zalacain M, Holmes DJ (2002) Novel Antibacterials: A Genomics Approach to Drug Discovery. Current Drug Targets—Infectious Disorders 2: 291–308. - PubMed
-
- Ehrt S, Schnappinger D (2006) Controlling Gene Expression in Mycobacteria. Future Microbiology 1: 177–184. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

