Abnormalities of Endocytosis, Phagocytosis, and Development Process in Dictyostelium Cells That Over-Express Acanthamoeba castellanii Metacaspase Protein
- PMID: 26246819
- PMCID: PMC4522297
Abnormalities of Endocytosis, Phagocytosis, and Development Process in Dictyostelium Cells That Over-Express Acanthamoeba castellanii Metacaspase Protein
Abstract
Background: Acanthamoeba castellanii forms a resistant cyst that protects the parasite against the host's immune response. Acanthamoeba Type-I metacaspase (Acmcp) is a caspase-like protein that has been found to be expressed during the encystations. Dictyostelium discoideum is an organism closely related to Acanthamoeba useful for studying the molecular function of this protozoan caspase-like protein.
Methods: The full length of Acmcp and a mutated version of the same gene, which lacks the proline rich N-terminal region (Acmcp-dpr), were cloned into the pDneo2a-GFP vector separately. The pDneo2a-GFP-Acmcp and pDneo2a-GFPAcmcp-dpr were electro-transfected into wild type D. discoideum cells to create cell lines that over-expressed Acmcp or Acmcp-dpr.
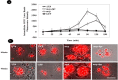
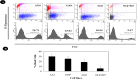
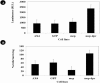
Results: Both cell lines that over-expressed Acmcp and Acmcp-dpr showed a significant increase in the fluid phase internalization and phagocytosis rate compared to the control cells. Additionally, the cells expressing the Acmcp-dpr mutant were unable to initiate early development and failed to aggregate or form fruiting bodies under starvation conditions, whereas Acmcp over-expressing cells showed the opposite phenomena. Quantitative cell death analysis provided additional support for these findings.
Conclusion: Acmcp is involved in the processes of endocytosis and phagocytosis. In addition, the proline rich region in Acmcp is important for cellular development in Dictyostelium. Given its important role in the development process, metacaspase protein is proposed as a candidate drug target against infections caused by A. castellanii.
Keywords: Acanthamoeba; Development; Dictyostelium; Metacaspase; Phagocytosis.
Figures




Similar articles
-
An Acanthamoeba castellanii metacaspase associates with the contractile vacuole and functions in osmoregulation.Exp Parasitol. 2013 Mar;133(3):314-26. doi: 10.1016/j.exppara.2012.12.001. Epub 2012 Dec 27. Exp Parasitol. 2013. PMID: 23274641
-
Caspase-like proteins: Acanthamoeba castellanii metacaspase and Dictyostelium discoideum paracaspase, what are their functions?J Biosci. 2014 Dec;39(5):909-16. doi: 10.1007/s12038-014-9486-0. J Biosci. 2014. PMID: 25431419 Review.
-
A type-1 metacaspase from Acanthamoeba castellanii.Microbiol Res. 2008;163(4):414-23. doi: 10.1016/j.micres.2006.06.017. Epub 2006 Aug 4. Microbiol Res. 2008. PMID: 16891103
-
Dictyostelium discoideum: a new host model system for intracellular pathogens of the genus Legionella.Cell Microbiol. 2000 Apr;2(2):165-71. doi: 10.1046/j.1462-5822.2000.00044.x. Cell Microbiol. 2000. PMID: 11207573
-
Acanthamoeba and Dictyostelium as Cellular Models for Legionella Infection.Front Cell Infect Microbiol. 2018 Mar 2;8:61. doi: 10.3389/fcimb.2018.00061. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29552544 Free PMC article. Review.
Cited by
-
Biological characteristics and pathogenicity of Acanthamoeba.Front Microbiol. 2023 Apr 5;14:1147077. doi: 10.3389/fmicb.2023.1147077. eCollection 2023. Front Microbiol. 2023. PMID: 37089530 Free PMC article. Review.
-
Roles of CgEde1 and CgMca in Development and Virulence of Colletotrichum gloeosporioides.Int J Mol Sci. 2024 Mar 3;25(5):2943. doi: 10.3390/ijms25052943. Int J Mol Sci. 2024. PMID: 38474190 Free PMC article.
References
-
- Di Gregorio C, Rivasi F, Mongiardo N, De Rienzo B, Wallace S, Visvesvara GS. Acanthamoeba meningoencephalitis in a patient with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1992; 116 ( 12): 1363– 5. - PubMed
-
- Uren AG, O'Rourke K, Aravind LA, Pisabarro MT, Seshagiri S, Koonin EV, Dixit VM. Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol Cell. 2000; 6 ( 4): 961– 7. - PubMed
-
- Mottram JC, Helms MJ, Coombs GH, Sajid M. Clan CD cysteine peptidases of parasitic protozoa. Trends Parasitol. 2003; 19: 182– 187. - PubMed
LinkOut - more resources
Full Text Sources
