Immune-mediated pathology in Duchenne muscular dystrophy
- PMID: 26246170
- PMCID: PMC5951380
- DOI: 10.1126/scitranslmed.aaa7322
Immune-mediated pathology in Duchenne muscular dystrophy
Abstract
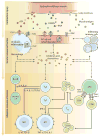
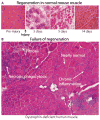
Immunological and inflammatory processes downstream of dystrophin deficiency as well as metabolic abnormalities, defective autophagy, and loss of regenerative capacity all contribute to muscle pathology in Duchenne muscular dystrophy (DMD). These downstream cascades offer potential avenues for pharmacological intervention. Modulating the inflammatory response and inducing immunological tolerance to de novo dystrophin expression will be critical to the success of dystrophin-replacement therapies. This Review focuses on the role of the inflammatory response in DMD pathogenesis and opportunities for clinical intervention.
Copyright © 2015, American Association for the Advancement of Science.
Figures
Similar articles
-
Skeletal Muscle Differentiation on a Chip Shows Human Donor Mesoangioblasts' Efficiency in Restoring Dystrophin in a Duchenne Muscular Dystrophy Model.Stem Cells Transl Med. 2016 Dec;5(12):1676-1683. doi: 10.5966/sctm.2015-0053. Epub 2016 Aug 8. Stem Cells Transl Med. 2016. PMID: 27502519 Free PMC article.
-
Pharmacological prospects in the treatment of Duchenne muscular dystrophy.Curr Opin Neurol. 2013 Oct;26(5):577-84. doi: 10.1097/WCO.0b013e328364fbaf. Curr Opin Neurol. 2013. PMID: 23995279 Review.
-
Drug Discovery of Therapies for Duchenne Muscular Dystrophy.J Biomol Screen. 2015 Dec;20(10):1189-203. doi: 10.1177/1087057115586535. Epub 2015 May 14. J Biomol Screen. 2015. PMID: 25975656 Review.
-
Alterations in Notch signalling in skeletal muscles from mdx and dko dystrophic mice and patients with Duchenne muscular dystrophy.Exp Physiol. 2014 Apr;99(4):675-87. doi: 10.1113/expphysiol.2013.077255. Epub 2014 Jan 17. Exp Physiol. 2014. PMID: 24443351
-
The role of branched fibres in the pathogenesis of Duchenne muscular dystrophy.Exp Physiol. 2011 Jun;96(6):564-71. doi: 10.1113/expphysiol.2010.056713. Epub 2011 Mar 18. Exp Physiol. 2011. PMID: 21421700 Review.
Cited by
-
High mobility group box 1 (HMGB1) is a potential disease biomarker in cell and mouse models of Duchenne muscular dystrophy.Biol Open. 2024 Sep 15;13(9):bio060542. doi: 10.1242/bio.060542. Epub 2024 Sep 5. Biol Open. 2024. PMID: 39158383 Free PMC article.
-
Report of a TREAT-NMD/World Duchenne Organisation Meeting on Dystrophin Quantification Methodology.J Neuromuscul Dis. 2019;6(1):147-159. doi: 10.3233/JND-180357. J Neuromuscul Dis. 2019. PMID: 30614809 Free PMC article.
-
CD38-NADase is a new major contributor to Duchenne muscular dystrophic phenotype.EMBO Mol Med. 2022 May 9;14(5):e12860. doi: 10.15252/emmm.202012860. Epub 2022 Mar 17. EMBO Mol Med. 2022. PMID: 35298089 Free PMC article.
-
Phase IIa trial in Duchenne muscular dystrophy shows vamorolone is a first-in-class dissociative steroidal anti-inflammatory drug.Pharmacol Res. 2018 Oct;136:140-150. doi: 10.1016/j.phrs.2018.09.007. Epub 2018 Sep 13. Pharmacol Res. 2018. PMID: 30219580 Free PMC article. Clinical Trial.
-
Mouse models of NADK2 deficiency analyzed for metabolic and gene expression changes to elucidate pathophysiology.Hum Mol Genet. 2022 Nov 28;31(23):4055-4074. doi: 10.1093/hmg/ddac151. Hum Mol Genet. 2022. PMID: 35796562 Free PMC article.
References
-
- National Center for Immunization, Respiratory Diseases. General recommendations on immunization—Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60:1–64. - PubMed
-
- Pétrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: A danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. - PubMed
-
- Hyldahl RD, Nelson B, Xin L, Welling T, Groscost L, Hubal MJ, Chipkin S, Clarkson PM, Parcell AC. Extracellular matrix remodeling and its contribution to protective adaptation following lengthening contractions in human muscle. FASEB J. 2015;29:2894–2904. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

