CD163 interacts with TWEAK to regulate tissue regeneration after ischaemic injury
- PMID: 26242746
- PMCID: PMC4918310
- DOI: 10.1038/ncomms8792
CD163 interacts with TWEAK to regulate tissue regeneration after ischaemic injury
Abstract
Macrophages are an essential component of the immune response to ischaemic injury and play an important role in promoting inflammation and its resolution, which is necessary for tissue repair. The type I transmembrane glycoprotein CD163 is exclusively expressed on macrophages, where it acts as a receptor for haemoglobin:haptoglobin complexes. An extracellular portion of CD163 circulates in the blood as a soluble protein, for which no physiological function has so far been described. Here we show that during ischaemia, soluble CD163 functions as a decoy receptor for TWEAK, a secreted pro-inflammatory cytokine of the tumour necrosis factor family, to regulate TWEAK-induced activation of canonical nuclear factor-κB (NF-κB) and Notch signalling necessary for myogenic progenitor cell proliferation. Mice with deletion of CD163 have transiently elevated levels of TWEAK, which stimulate muscle satellite cell proliferation and tissue regeneration in their ischaemic and non-ischaemic limbs. These results reveal a role for soluble CD163 in regulating muscle regeneration after ischaemic injury.
Figures
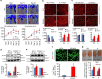
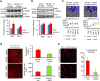
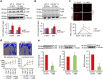
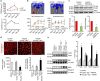
 P<0.05 versus other groups in d,e. Comparisons between groups were achieved using a two-sided student's t-test. For multiple group comparisons, we utilized a one-way ANOVA. If the variance ratio test (F-test) was significant, a more detailed post hoc analysis of differences between groups was made using a Tukey–Kramer honest significance difference test.
P<0.05 versus other groups in d,e. Comparisons between groups were achieved using a two-sided student's t-test. For multiple group comparisons, we utilized a one-way ANOVA. If the variance ratio test (F-test) was significant, a more detailed post hoc analysis of differences between groups was made using a Tukey–Kramer honest significance difference test.

Similar articles
-
The TWEAK/Fn14/CD163 axis-implications for metabolic disease.Rev Endocr Metab Disord. 2022 Jun;23(3):449-462. doi: 10.1007/s11154-021-09688-4. Epub 2021 Sep 20. Rev Endocr Metab Disord. 2022. PMID: 34542797 Free PMC article. Review.
-
A previously unrecognized protein-protein interaction between TWEAK and CD163: potential biological implications.J Immunol. 2007 Jun 15;178(12):8183-94. doi: 10.4049/jimmunol.178.12.8183. J Immunol. 2007. PMID: 17548657
-
The CD163-expressing macrophages recognize and internalize TWEAK: potential consequences in atherosclerosis.Atherosclerosis. 2009 Nov;207(1):103-10. doi: 10.1016/j.atherosclerosis.2009.04.033. Epub 2009 May 4. Atherosclerosis. 2009. PMID: 19473660
-
TWEAK, via its receptor Fn14, is a novel regulator of mesenchymal progenitor cells and skeletal muscle regeneration.EMBO J. 2006 Dec 13;25(24):5826-39. doi: 10.1038/sj.emboj.7601441. Epub 2006 Nov 23. EMBO J. 2006. PMID: 17124496 Free PMC article.
-
The macrophage scavenger receptor CD163.Immunobiology. 2005;210(2-4):153-60. doi: 10.1016/j.imbio.2005.05.010. Immunobiology. 2005. PMID: 16164022 Review.
Cited by
-
Crossroads between peripheral atherosclerosis, western-type diet and skeletal muscle pathophysiology: emphasis on apolipoprotein E deficiency and peripheral arterial disease.J Biomed Sci. 2017 Jul 8;24(1):42. doi: 10.1186/s12929-017-0346-8. J Biomed Sci. 2017. PMID: 28688452 Free PMC article. Review.
-
The Role of Soluble CD163 (sCD163) in Human Physiology and Pathophysiology.Cells. 2024 Oct 11;13(20):1679. doi: 10.3390/cells13201679. Cells. 2024. PMID: 39451197 Free PMC article. Review.
-
The TWEAK/Fn14/CD163 axis-implications for metabolic disease.Rev Endocr Metab Disord. 2022 Jun;23(3):449-462. doi: 10.1007/s11154-021-09688-4. Epub 2021 Sep 20. Rev Endocr Metab Disord. 2022. PMID: 34542797 Free PMC article. Review.
-
Precision engineering for PRRSV resistance in pigs: Macrophages from genome edited pigs lacking CD163 SRCR5 domain are fully resistant to both PRRSV genotypes while maintaining biological function.PLoS Pathog. 2017 Feb 23;13(2):e1006206. doi: 10.1371/journal.ppat.1006206. eCollection 2017 Feb. PLoS Pathog. 2017. PMID: 28231264 Free PMC article.
-
CD163 protein inhibits lipopolysaccharide-induced macrophage transformation from M2 to M1 involved in disruption of the TWEAK-Fn14 interaction.Heliyon. 2023 Dec 4;10(1):e23223. doi: 10.1016/j.heliyon.2023.e23223. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38148798 Free PMC article.
References
-
- Lloyd-Jones D. et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 119, 480–486 (2009). - PubMed
-
- Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 3, 23–35 (2003). - PubMed
-
- Kristiansen M. et al. Identification of the haemoglobin scavenger receptor. Nature 409, 198–201 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

