Mouse Model of Human Congenital Heart Disease: Progressive Atrioventricular Block Induced by a Heterozygous Nkx2-5 Homeodomain Missense Mutation
- PMID: 26226998
- PMCID: PMC4618020
- DOI: 10.1161/CIRCEP.115.002720
Mouse Model of Human Congenital Heart Disease: Progressive Atrioventricular Block Induced by a Heterozygous Nkx2-5 Homeodomain Missense Mutation
Abstract
Background: Heterozygous human NKX2-5 homeodomain (DNA-binding domain) missense mutations are highly penetrant for varied congenital heart defects, including progressive atrioventricular (AV) block requiring pacemaker implantation. We recently replicated this genetic defect in a murine knockin model, in which we demonstrated highly penetrant, pleiotropic cardiac anomalies. In this study, we examined postnatal AV conduction in the knockin mice.
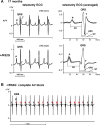
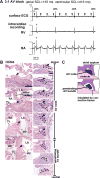
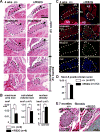
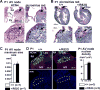
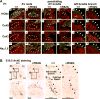
Methods and results: A murine knockin model (Arg52Gly, Nkx2-5(+/R52G)) in a 129/Sv background was analyzed by histopathology, surface, and telemetry ECG, and in vivo electrophysiology studies, comparing with control Nkx2-5(+/+) mice at diverse postnatal stages, ranging from postnatal day 1 (P1) to 17 months. PR prolongation (first degree AV block) was present at 4 weeks, 7 months, and 17 months of age, but not at P1 in the mutant mice. Advanced AV block was also occasionally demonstrated in the mutant mice. Electrophysiology studies showed that AV nodal function and right ventricular effective refractory period were impaired in the mutant mice, whereas sinus nodal function was not affected. AV nodal size was significantly smaller in the mutant mice than their controls at 4 weeks of age, corresponding to the presence of PR prolongation, but not P1, suggesting, at least in part, that the conduction abnormalities are the result of a morphologically atrophic AV node.
Conclusions: The highly penetrant and progressive AV block phenotype seen in human heterozygous missense mutations in NKX2-5 homeodomain was replicated in mice by knocking in a comparable missense mutation.
Keywords: animal models; atrioventricular block; congenital heart defects; genetics; human.
© 2015 American Heart Association, Inc.
Conflict of interest statement
Figures

Similar articles
-
A mouse model of human congenital heart disease: high incidence of diverse cardiac anomalies and ventricular noncompaction produced by heterozygous Nkx2-5 homeodomain missense mutation.Circ Cardiovasc Genet. 2014 Aug;7(4):423-433. doi: 10.1161/CIRCGENETICS.113.000281. Epub 2014 Jul 15. Circ Cardiovasc Genet. 2014. PMID: 25028484 Free PMC article.
-
Progressive atrioventricular conduction defects and heart failure in mice expressing a mutant Csx/Nkx2.5 homeoprotein.J Clin Invest. 2001 Jul;108(2):189-201. doi: 10.1172/JCI12694. J Clin Invest. 2001. PMID: 11457872 Free PMC article.
-
Biochemical analyses of eight NKX2.5 homeodomain missense mutations causing atrioventricular block and cardiac anomalies.Cardiovasc Res. 2004 Oct 1;64(1):40-51. doi: 10.1016/j.cardiores.2004.06.004. Cardiovasc Res. 2004. PMID: 15364612
-
Defects in cardiac conduction system lineages and malignant arrhythmias: developmental pathways and disease.Novartis Found Symp. 2003;250:260-70; discussion 271-5, 276-9. Novartis Found Symp. 2003. PMID: 12956335 Review.
-
Function follows form: cardiac conduction system defects in Nkx2-5 mutation.Anat Rec A Discov Mol Cell Evol Biol. 2004 Oct;280(2):966-72. doi: 10.1002/ar.a.20102. Anat Rec A Discov Mol Cell Evol Biol. 2004. PMID: 15368343 Review.
Cited by
-
Association between single-nucleotide polymorphisms of NKX2.5 and congenital heart disease in Chinese population: A meta-analysis.Open Life Sci. 2022 May 12;17(1):473-482. doi: 10.1515/biol-2022-0058. eCollection 2022. Open Life Sci. 2022. PMID: 35647298 Free PMC article.
-
Genetics of congenital heart disease: a narrative review of recent advances and clinical implications.Transl Pediatr. 2021 Sep;10(9):2366-2386. doi: 10.21037/tp-21-297. Transl Pediatr. 2021. PMID: 34733677 Free PMC article. Review.
-
New Insights into the Development and Morphogenesis of the Cardiac Purkinje Fiber Network: Linking Architecture and Function.J Cardiovasc Dev Dis. 2021 Aug 7;8(8):95. doi: 10.3390/jcdd8080095. J Cardiovasc Dev Dis. 2021. PMID: 34436237 Free PMC article. Review.
-
Linagliptin and secoisolariciresinol diglucoside attenuate hyperlipidemia and cardiac hypertrophy induced by a high-methionine diet in rats via suppression of hyperhomocysteinemia-induced endoplasmic reticulum stress.Front Pharmacol. 2023 Nov 9;14:1275730. doi: 10.3389/fphar.2023.1275730. eCollection 2023. Front Pharmacol. 2023. PMID: 38026992 Free PMC article.
-
Personalized Genetic Diagnosis of Congenital Heart Defects in Newborns.J Pers Med. 2021 Jun 16;11(6):562. doi: 10.3390/jpm11060562. J Pers Med. 2021. PMID: 34208491 Free PMC article. Review.
References
-
- Benson DW, Silberbach GM, Kavanaugh-McHugh A, Cottrill C, Zhang Y, Riggs S, Smalls O, Johnson MC, Watson MS, Seidman JG, Seidman CE, Plowden J, Kugler JD. Mutations in the cardiac transcription factor nkx2.5 affect diverse cardiac developmental pathways. J Clin Invest. 1999;104:1567–1573. - PMC - PubMed
-
- Reamon-Buettner SM, Borlak J. Nkx2-5: An update on this hypermutable homeodomain protein and its role in human congenital heart disease (chd) Hum Mutat. 2010;31:1185–1194. - PubMed
-
- Stallmeyer B, Fenge H, Nowak-Gottl U, Schulze-Bahr E. Mutational spectrum in the cardiac transcription factor gene nkx2.5 (csx) associated with congenital heart disease. Clin Genet. 2010;78:533–540. - PubMed
-
- De Luca A, Sarkozy A, Consoli F, Ferese R, Guida V, Dentici ML, Mingarelli R, Bellacchio E, Tuo G, Limongelli G, Digilio MC, Marino B, Dallapiccola B. Familial transposition of the great arteries caused by multiple mutations in laterality genes. Heart. 2010;96:673–677. - PubMed
-
- Liu XY, Wang J, Yang YQ, Zhang YY, Chen XZ, Zhang W, Wang XZ, Zheng JH, Chen YH. Novel Nkx2-5 mutations in patients with familial atrial septal defects. Pediatr Cardiol. 2011;32:193–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

