Left-Biased Spermatogenic Failure in 129/SvJ Dnd1Ter/+ Mice Correlates with Differences in Vascular Architecture, Oxygen Availability, and Metabolites
- PMID: 26224005
- PMCID: PMC6322448
- DOI: 10.1095/biolreprod.115.128850
Left-Biased Spermatogenic Failure in 129/SvJ Dnd1Ter/+ Mice Correlates with Differences in Vascular Architecture, Oxygen Availability, and Metabolites
Abstract
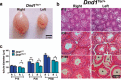
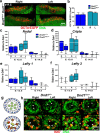
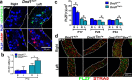
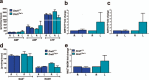
Homozygosity for the Ter mutation in the RNA-binding protein Dead end 1 (Dnd1(Ter/Ter)) sensitizes germ cells to degeneration in all mouse strains. In 129/SvJ mice, approximately 10% of Dnd1(Ter/+) heterozygotes develop spermatogenic failure, and 95% of unilateral cases occur in the left testis. The first differences between right and left testes were detected at Postnatal Day 15 when many more spermatogonial stem cells (SSCs) were undergoing apoptosis in the left testis compared to the right. As we detected no significant left/right differences in the molecular pathway associated with body axis asymmetry or in the expression of signals known to promote proliferation, differentiation, and survival of germ cells, we investigated whether physiological differences might account for asymmetry of the degeneration phenotype. We show that left/right differences in vascular architecture are associated with a decrease in hemoglobin saturation and increased levels of HIF-1alpha in the left testis compared to the right. In Dnd1 heterozygotes, lower oxygen availability was associated with metabolic differences, including lower levels of ATP and NADH in the left testis. These experiments suggest a dependence on oxygen availability and metabolic substrates for SSC survival and suggest that Dnd1(Ter/+) SSCs may act as efficient sensors to detect subtle environmental changes that alter SSC fate.
Keywords: Dnd1/DND1; germ cell; hypoxia; metabolism; spermatogenic failure; spermatogonial stem cells.
© 2015 by the Society for the Study of Reproduction, Inc.
Figures


Similar articles
-
Oxygen availability influences the incidence of testicular teratoma in Dnd1Ter/+ mice.Front Genet. 2023 Apr 26;14:1179256. doi: 10.3389/fgene.2023.1179256. eCollection 2023. Front Genet. 2023. PMID: 37180974 Free PMC article.
-
Spermatogonial depletion in adult Pin1-deficient mice.Biol Reprod. 2003 Dec;69(6):1989-97. doi: 10.1095/biolreprod.103.020859. Epub 2003 Aug 20. Biol Reprod. 2003. PMID: 12930711
-
Essential role of mouse Dead end1 in the maintenance of spermatogonia.Dev Biol. 2019 Jan 1;445(1):103-112. doi: 10.1016/j.ydbio.2018.11.003. Epub 2018 Nov 12. Dev Biol. 2019. PMID: 30439356
-
[The sertoli cells, testicular differentiation and male infertility].Akush Ginekol (Sofiia). 2012;51 Suppl 1:15-8. Akush Ginekol (Sofiia). 2012. PMID: 23236673 Review. Bulgarian. No abstract available.
-
Molecular regulation of spermatogonial stem cell renewal and differentiation.Reproduction. 2019 Nov;158(5):R169-R187. doi: 10.1530/REP-18-0476. Reproduction. 2019. PMID: 31247585 Review.
Cited by
-
WOMEN IN REPRODUCTIVE SCIENCE: To be or not to be a testis.Reproduction. 2019 Dec;158(6):F101-F111. doi: 10.1530/REP-19-0151. Reproduction. 2019. PMID: 31265995 Free PMC article. Review.
-
The hypoxia-inducible factor EPAS1 is required for spermatogonial stem cell function in regenerative conditions.iScience. 2023 Nov 9;26(12):108424. doi: 10.1016/j.isci.2023.108424. eCollection 2023 Dec 15. iScience. 2023. PMID: 38077147 Free PMC article.
-
Editorial: New insights in cellular and molecular biology of cancer stem cells.Front Genet. 2023 Oct 2;14:1297971. doi: 10.3389/fgene.2023.1297971. eCollection 2023. Front Genet. 2023. PMID: 37849502 Free PMC article. No abstract available.
-
Testicular abnormalities in mice with Y chromosome deficiencies.Biol Reprod. 2017 Mar 1;96(3):694-706. doi: 10.1095/biolreprod.116.144006. Biol Reprod. 2017. PMID: 28339606 Free PMC article.
-
Parent-of-origin effects of A1CF and AGO2 on testicular germ-cell tumors, testicular abnormalities, and fertilization bias.Proc Natl Acad Sci U S A. 2016 Sep 13;113(37):E5425-33. doi: 10.1073/pnas.1604773113. Epub 2016 Aug 31. Proc Natl Acad Sci U S A. 2016. PMID: 27582469 Free PMC article.
References
-
- De Kretser DM, Baker HWG.. Infertility in men: recent advances and continuing controversies. J Clin Endocrinol Metab 1999; 84:3443–3450. - PubMed
-
- Berookhim BM, Schlegel PN.. Azoospermia due to spermatogenic failure. Urol Clin N Am 2014; 41:97–113. - PubMed
-
- Krausz C. Male infertility: pathogenesis and clinical diagnosis. Best Pract Res Clin Endocrinol Metab 2011; 25:271–285. - PubMed
-
- Ginsburg M, Snow MH, McLaren A.. Primordial germ cells in the mouse embryo during gastrulation. Development 1990; 110:521–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

