The ecdysone receptor signalling regulates microvilli formation in follicular epithelial cells
- PMID: 26223269
- PMCID: PMC11108565
- DOI: 10.1007/s00018-015-1999-7
The ecdysone receptor signalling regulates microvilli formation in follicular epithelial cells
Abstract
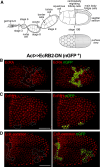
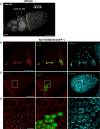
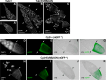
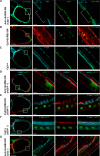
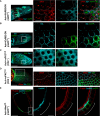
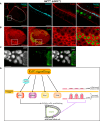
Epithelial morphogenesis contributes greatly to the development and homeostasis of the organs and body parts. Here, we analysed the consequences of impaired ecdysone receptor (EcR) signalling in the Drosophila follicular epithelium. Besides governing cell growth, the three EcR isoforms act redundantly in controlling follicle cell positioning. Flattening of the microvilli and an aberrant actin cytoskeleton arise from defective EcR signalling in follicle cells, and these defects impact on the organisation of the oocyte membrane. We found that this signalling governs a complex molecular network since its impairment affects key molecules as atypical protein kinase C and activated Moesin. Interestingly, the activity of the transcription factor Tramtrack69 isoform is required for microvilli and their actin core morphogenesis as well as for follicle cell positioning. In conclusion, our findings provide evidence of novel roles for EcR signalling and Tramtrack69 transcription factor in controlling stage-specific differentiation events that take place in the follicular epithelium.
Keywords: Cad99C; EcR dominant negative; Ecdysone signalling; Oogenesis.
Figures


Similar articles
-
Cell survival and polarity of Drosophila follicle cells require the activity of ecdysone receptor B1 isoform.Genetics. 2009 Jan;181(1):165-75. doi: 10.1534/genetics.108.096008. Epub 2008 Nov 17. Genetics. 2009. PMID: 19015542 Free PMC article.
-
Ras signaling modulates activity of the ecdysone receptor EcR during cell migration in the Drosophila ovary.Dev Dyn. 2007 May;236(5):1213-26. doi: 10.1002/dvdy.21140. Dev Dyn. 2007. PMID: 17436275
-
Steroid signaling in mature follicles is important for Drosophila ovulation.Proc Natl Acad Sci U S A. 2017 Jan 24;114(4):699-704. doi: 10.1073/pnas.1614383114. Epub 2017 Jan 9. Proc Natl Acad Sci U S A. 2017. PMID: 28069934 Free PMC article.
-
Myosin VIIA regulates microvillus morphogenesis and interacts with cadherin Cad99C in Drosophila oogenesis.J Cell Sci. 2014 Nov 15;127(Pt 22):4821-32. doi: 10.1242/jcs.099242. Epub 2014 Sep 18. J Cell Sci. 2014. PMID: 25236597
-
Ecdysone signalling and ovarian development in insects: from stem cells to ovarian follicle formation.Biochim Biophys Acta. 2015 Feb;1849(2):181-6. doi: 10.1016/j.bbagrm.2014.05.025. Epub 2014 Jun 3. Biochim Biophys Acta. 2015. PMID: 24939835 Review.
Cited by
-
Downregulation of homeodomain protein Cut is essential for Drosophila follicle maturation and ovulation.Development. 2019 Sep 19;146(18):dev179002. doi: 10.1242/dev.179002. Development. 2019. PMID: 31444217 Free PMC article.
-
Finishing the egg.Genetics. 2024 Jan 3;226(1):iyad183. doi: 10.1093/genetics/iyad183. Genetics. 2024. PMID: 38000906 Free PMC article.
-
Two phases for centripetal migration of Drosophila melanogaster follicle cells: initial ingression followed by epithelial migration.Development. 2023 Mar 15;150(6):dev200492. doi: 10.1242/dev.200492. Epub 2023 Mar 30. Development. 2023. PMID: 36807509 Free PMC article.
-
Nuclear receptors linking physiology and germline stem cells in Drosophila.Vitam Horm. 2021;116:327-362. doi: 10.1016/bs.vh.2020.12.008. Vitam Horm. 2021. PMID: 33752824 Free PMC article. Review.
-
Steroid Hormones and the Physiological Regulation of Tissue-Resident Stem Cells: Lessons from the Drosophila Ovary.Curr Stem Cell Rep. 2017 Mar;3(1):9-18. doi: 10.1007/s40778-017-0070-z. Epub 2017 Feb 1. Curr Stem Cell Rep. 2017. PMID: 28458991 Free PMC article.
References
-
- Bownes M. The roles of juvenile hormone, ecdysone and the ovary in the control of Drosophila vitellogenesis. J Insect Physiol. 1989;34:409–413. doi: 10.1016/0022-1910(89)90115-7. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

