Pli1(PIAS1) SUMO ligase protected by the nuclear pore-associated SUMO protease Ulp1SENP1/2
- PMID: 26221037
- PMCID: PMC4566240
- DOI: 10.1074/jbc.M115.673038
Pli1(PIAS1) SUMO ligase protected by the nuclear pore-associated SUMO protease Ulp1SENP1/2
Abstract
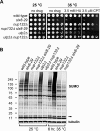
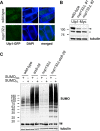
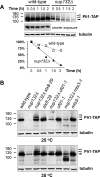
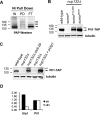
Covalent modification of the proteome by SUMO is critical for genetic stability and cell growth. Equally crucial to these processes is the removal of SUMO from its targets by the Ulp1 (HuSENP1/2) family of SUMO proteases. Ulp1 activity is normally spatially restricted, because it is localized to the nuclear periphery via interactions with the nuclear pore. Delocalization of Ulp1 causes DNA damage and cell cycle defects, phenotypes thought to be caused by inappropriate desumoylation of nucleoplasmic targets that are normally spatially protected from Ulp1. Here, we define a novel consequence of Ulp1 deregulation, with a major impact on SUMO pathway function. In fission yeast lacking Nup132 (Sc/HuNUP133), Ulp1 is delocalized and can no longer antagonize sumoylation of the PIAS family SUMO E3 ligase, Pli1. Consequently, SUMO chain-modified Pli1 is targeted for proteasomal degradation by the concerted action of a SUMO-targeted ubiquitin ligase (STUbL) and Cdc48-Ufd1-Npl4. Pli1 degradation causes the profound SUMO pathway defects and associated centromere dysfunction in cells lacking Nup132. Thus, perhaps counterintuitively, Ulp1-mediated desumoylation can promote SUMO modification by stabilizing a SUMO E3 ligase.
Keywords: Cdc48-Ufd1-Npl4; Pli1; STUbL; Ulp1; nuclear pore; proteasome; protein degradation; small ubiquitin-like modifier (SUMO); sumoylation.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
Concerted action of the ubiquitin-fusion degradation protein 1 (Ufd1) and Sumo-targeted ubiquitin ligases (STUbLs) in the DNA-damage response.PLoS One. 2013 Nov 12;8(11):e80442. doi: 10.1371/journal.pone.0080442. eCollection 2013. PLoS One. 2013. PMID: 24265825 Free PMC article.
-
Dual recruitment of Cdc48 (p97)-Ufd1-Npl4 ubiquitin-selective segregase by small ubiquitin-like modifier protein (SUMO) and ubiquitin in SUMO-targeted ubiquitin ligase-mediated genome stability functions.J Biol Chem. 2012 Aug 24;287(35):29610-9. doi: 10.1074/jbc.M112.379768. Epub 2012 Jun 22. J Biol Chem. 2012. PMID: 22730331 Free PMC article.
-
The nuclear pore primes recombination-dependent DNA synthesis at arrested forks by promoting SUMO removal.Nat Commun. 2020 Nov 6;11(1):5643. doi: 10.1038/s41467-020-19516-z. Nat Commun. 2020. PMID: 33159083 Free PMC article.
-
The role of Schizosaccharomyces pombe SUMO ligases in genome stability.Biochem Soc Trans. 2007 Dec;35(Pt 6):1379-84. doi: 10.1042/BST0351379. Biochem Soc Trans. 2007. PMID: 18031226 Review.
-
Cytoplasmic sumoylation by PIAS-type Siz1-SUMO ligase.Cell Cycle. 2008 Jun 15;7(12):1738-44. doi: 10.4161/cc.7.12.6156. Epub 2008 Jun 16. Cell Cycle. 2008. PMID: 18583943 Review.
Cited by
-
SUMO-Based Regulation of Nuclear Positioning to Spatially Regulate Homologous Recombination Activities at Replication Stress Sites.Genes (Basel). 2021 Dec 17;12(12):2010. doi: 10.3390/genes12122010. Genes (Basel). 2021. PMID: 34946958 Free PMC article. Review.
-
SUMO-targeted ubiquitin ligase activity can either suppress or promote genome instability, depending on the nature of the DNA lesion.PLoS Genet. 2017 May 5;13(5):e1006776. doi: 10.1371/journal.pgen.1006776. eCollection 2017 May. PLoS Genet. 2017. PMID: 28475613 Free PMC article.
-
Recruitment of a SUMO isopeptidase to rDNA stabilizes silencing complexes by opposing SUMO targeted ubiquitin ligase activity.Genes Dev. 2017 Apr 15;31(8):802-815. doi: 10.1101/gad.296145.117. Epub 2017 May 9. Genes Dev. 2017. PMID: 28487408 Free PMC article.
-
A reference-based protein degradation assay without global translation inhibitors.J Biol Chem. 2017 Dec 29;292(52):21457-21465. doi: 10.1074/jbc.M117.814236. Epub 2017 Nov 9. J Biol Chem. 2017. PMID: 29122887 Free PMC article.
-
SUMO targeting of a stress-tolerant Ulp1 SUMO protease.PLoS One. 2018 Jan 19;13(1):e0191391. doi: 10.1371/journal.pone.0191391. eCollection 2018. PLoS One. 2018. PMID: 29351565 Free PMC article.
References
-
- Girdwood D. W., Tatham M. H., Hay R. T. (2004) SUMO and transcriptional regulation. Semin. Cell Dev. Biol. 15, 201–210 - PubMed
-
- Jackson S. P., Durocher D. (2013) Regulation of DNA damage responses by ubiquitin and SUMO. Mol. Cell 49, 795–807 - PubMed
-
- Kerscher O., Felberbaum R., Hochstrasser M. (2006) Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell Dev. Biol. 22, 159–180 - PubMed
-
- Felberbaum R., Hochstrasser M. (2008) Ulp2 and the DNA damage response: desumoylation enables safe passage through mitosis. Cell Cycle 7, 52–56 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

