Fecal microbiota transplantation and bacterial consortium transplantation have comparable effects on the re-establishment of mucosal barrier function in mice with intestinal dysbiosis
- PMID: 26217323
- PMCID: PMC4493656
- DOI: 10.3389/fmicb.2015.00692
Fecal microbiota transplantation and bacterial consortium transplantation have comparable effects on the re-establishment of mucosal barrier function in mice with intestinal dysbiosis
Abstract
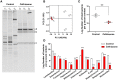
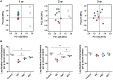
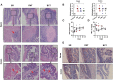
Fecal microbiota transplantation (FMT) is a promising therapy, despite some reports of adverse side effects. Bacterial consortia transplantation (BCT) for targeted restoration of the intestinal ecosystem is considered a relatively safe and simple procedure. However, no systematic research has assessed the effects of FMT and BCT on immune responses of intestinal mucosal barrier in patients. We conducted complementary studies in animal models on the effects of FMT and BCT, and provide recommendations for improving the clinical outcomes of these treatments. To establish the dysbiosis model, male BALB/c mice were treated with ceftriaxone intra-gastrically for 7 days. After that, FMT and BCT were performed on ceftriaxone-treated mice for 3 consecutive days to rebuild the intestinal ecosystem. Post-FMT and post-BCT changes of the intestinal microbial community and mucosal barrier functions were investigated and compared. Disruption of intestinal microbial homeostasis impacted the integrity of mucosal epithelial layer, resulting in increased intestinal permeability. These outcomes were accompanied by overexpression of Muc2, significant decrease of SIgA secretion, and overproduction of defensins and inflammatory cytokines. After FMT and BCT, the intestinal microbiota recovered quickly, this was associated with better reconstruction of mucosal barriers and re-establishment of immune networks compared with spontaneous recovery (SR). Although based on a short-term study, our results suggest that FMT and BCT promote the re-establishment of intestinal microbial communities in mice with antibiotic-induced dysbiosis, and contribute to the temporal and spatial interactions between microbiota and mucosal barriers. The effects of BCT are comparable to that of FMT, especially in normalizing the intestinal levels of Muc2, SIgA, and defensins.
Keywords: bacterial consortia transplantation; fecal microbiota transplantation; intestinal dysbiosis; intestinal microbiota; mucosal barrier function.
Figures



Similar articles
-
Fecal Microbiota Transplantation Beneficially Regulates Intestinal Mucosal Autophagy and Alleviates Gut Barrier Injury.mSystems. 2018 Oct 9;3(5):e00137-18. doi: 10.1128/mSystems.00137-18. eCollection 2018 Sep-Oct. mSystems. 2018. PMID: 30320222 Free PMC article.
-
Fecal Microbiota Transplantation Protects the Intestinal Mucosal Barrier by Reconstructing the Gut Microbiota in a Murine Model of Sepsis.Front Cell Infect Microbiol. 2021 Sep 22;11:736204. doi: 10.3389/fcimb.2021.736204. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34631604 Free PMC article.
-
Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis.Inflamm Bowel Dis. 2013 Sep;19(10):2155-65. doi: 10.1097/MIB.0b013e31829ea325. Inflamm Bowel Dis. 2013. PMID: 23899544
-
Treating From the Inside Out: Relevance of Fecal Microbiota Transplantation to Counteract Gut Damage in GVHD and HIV Infection.Front Med (Lausanne). 2020 Aug 6;7:421. doi: 10.3389/fmed.2020.00421. eCollection 2020. Front Med (Lausanne). 2020. PMID: 32850913 Free PMC article. Review.
-
Standardized Preparation for Fecal Microbiota Transplantation in Pigs.Front Microbiol. 2018 Jun 19;9:1328. doi: 10.3389/fmicb.2018.01328. eCollection 2018. Front Microbiol. 2018. PMID: 29971061 Free PMC article. Review.
Cited by
-
The Anti-Inflammatory Effect and Mucosal Barrier Protection of Clostridium butyricum RH2 in Ceftriaxone-Induced Intestinal Dysbacteriosis.Front Cell Infect Microbiol. 2021 Mar 25;11:647048. doi: 10.3389/fcimb.2021.647048. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33842393 Free PMC article.
-
The human microbiome and juvenile idiopathic arthritis.Pediatr Rheumatol Online J. 2016 Sep 20;14(1):55. doi: 10.1186/s12969-016-0114-4. Pediatr Rheumatol Online J. 2016. PMID: 27650128 Free PMC article. Review.
-
Any Future for Faecal Microbiota Transplantation as a Novel Strategy for Gut Microbiota Modulation in Human and Veterinary Medicine?Life (Basel). 2022 May 12;12(5):723. doi: 10.3390/life12050723. Life (Basel). 2022. PMID: 35629390 Free PMC article. Review.
-
Tributyrin alleviates gut microbiota dysbiosis to repair intestinal damage in antibiotic-treated mice.PLoS One. 2023 Jul 31;18(7):e0289364. doi: 10.1371/journal.pone.0289364. eCollection 2023. PLoS One. 2023. PMID: 37523400 Free PMC article.
-
Suppression of gut dysbiosis reverses Western diet-induced vascular dysfunction.Am J Physiol Endocrinol Metab. 2018 May 1;314(5):E468-E477. doi: 10.1152/ajpendo.00187.2017. Epub 2017 Dec 26. Am J Physiol Endocrinol Metab. 2018. PMID: 29351482 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

