Kremen1 and Dickkopf1 control cell survival in a Wnt-independent manner
- PMID: 26206087
- PMCID: PMC4716294
- DOI: 10.1038/cdd.2015.100
Kremen1 and Dickkopf1 control cell survival in a Wnt-independent manner
Abstract
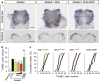
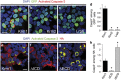
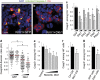
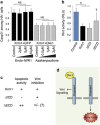
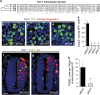
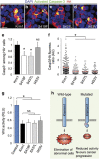
In multicellular organisms, a tight control of cell death is required to ensure normal development and tissue homeostasis. Improper function of apoptotic or survival pathways can not only affect developmental programs but also favor cancer progression. Here we describe a novel apoptotic signaling pathway involving the transmembrane receptor Kremen1 and its ligand, the Wnt-antagonist Dickkopf1. Using a whole embryo culture system, we first show that Dickkopf1 treatment promotes cell survival in a mouse model exhibiting increased apoptosis in the developing neural plate. Remarkably, this effect was not recapitulated by chemical Wnt inhibition. We then show that Dickkopf1 receptor Kremen1 is a bona fide dependence receptor, triggering cell death unless bound to its ligand. We performed Wnt-activity assays to demonstrate that the pro-apoptotic and anti-Wnt functions mediated by Kremen1 are strictly independent. Furthermore, we combined phylogenetic and mutagenesis approaches to identify a specific motif in the cytoplasmic tail of Kremen1, which is (i) specifically conserved in the lineage of placental mammals and (ii) strictly required for apoptosis induction. Finally, we show that somatic mutations of kremen1 found in human cancers can affect its pro-apoptotic activity, supporting a tumor suppressor function. Our findings thus reveal a new Wnt-independent function for Kremen1 and Dickkopf1 in the regulation of cell survival with potential implications in cancer therapies.
Figures

Similar articles
-
Kremen1-induced cell death is regulated by homo- and heterodimerization.Cell Death Discov. 2019 May 1;5:91. doi: 10.1038/s41420-019-0175-5. eCollection 2019. Cell Death Discov. 2019. PMID: 31069116 Free PMC article.
-
Kremen1 restricts Dkk activity during posterior lateral line development in zebrafish.Development. 2014 Aug;141(16):3212-21. doi: 10.1242/dev.102541. Epub 2014 Jul 18. Development. 2014. PMID: 25038040 Free PMC article.
-
Dickkopf1: An immunomodulatory ligand and Wnt antagonist in pathological inflammation.Differentiation. 2019 Jul-Aug;108:33-39. doi: 10.1016/j.diff.2019.05.003. Epub 2019 Jun 12. Differentiation. 2019. PMID: 31221431 Free PMC article. Review.
-
Function and biological roles of the Dickkopf family of Wnt modulators.Oncogene. 2006 Dec 4;25(57):7469-81. doi: 10.1038/sj.onc.1210054. Oncogene. 2006. PMID: 17143291 Review.
-
Dimer-dependent intrinsic/basal activity of the class B G protein-coupled receptor PAC1 promotes cellular anti-apoptotic activity through Wnt/β-catenin pathways that are associated with dimer endocytosis.PLoS One. 2014 Nov 26;9(11):e113913. doi: 10.1371/journal.pone.0113913. eCollection 2014. PLoS One. 2014. PMID: 25426938 Free PMC article.
Cited by
-
miRNA-431 Prevents Amyloid-β-Induced Synapse Loss in Neuronal Cell Culture Model of Alzheimer's Disease by Silencing Kremen1.Front Cell Neurosci. 2018 Mar 28;12:87. doi: 10.3389/fncel.2018.00087. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29643768 Free PMC article.
-
Dickkopf1 induces enteric neurogenesis and gliogenesis in vitro if apoptosis is evaded.Commun Biol. 2023 Aug 2;6(1):808. doi: 10.1038/s42003-023-05072-x. Commun Biol. 2023. PMID: 37532804 Free PMC article.
-
Molecular Insight Into the Therapeutic Potential of Long Non-coding RNA-Associated Competing Endogenous RNA Axes in Alzheimer's Disease: A Systematic Scoping Review.Front Aging Neurosci. 2021 Nov 25;13:742242. doi: 10.3389/fnagi.2021.742242. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34899268 Free PMC article.
-
Kremen1-induced cell death is regulated by homo- and heterodimerization.Cell Death Discov. 2019 May 1;5:91. doi: 10.1038/s41420-019-0175-5. eCollection 2019. Cell Death Discov. 2019. PMID: 31069116 Free PMC article.
-
Identification of a New Prediction Model for Bladder Cancer Related to Immune Functions and Chemotherapy Using Gene Sets of Biological Processes.Biomed Res Int. 2022 Oct 30;2022:4740686. doi: 10.1155/2022/4740686. eCollection 2022. Biomed Res Int. 2022. PMID: 36349315 Free PMC article.
References
-
- 1Goldschneider D, Mehlen P. Dependence receptors: a new paradigm in cell signaling and cancer therapy. Oncogene 2010; 29: 1865–1882. - PubMed
-
- 2Mehlen P, Rabizadeh S, Snipas SJ, Assa-Munt N, Salvesen GS, Bredesen DE. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature 1998; 395: 801–804. - PubMed
-
- 4Thibert C, Teillet MA, Lapointe F, Mazelin L, Le Douarin NM, Mehlen P. Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science 2003; 301: 843–846. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

