Innate cellular sources of interleukin-17A regulate macrophage accumulation in cigarette- smoke-induced lung inflammation in mice
- PMID: 26201093
- PMCID: PMC4613531
- DOI: 10.1042/CS20140703
Innate cellular sources of interleukin-17A regulate macrophage accumulation in cigarette- smoke-induced lung inflammation in mice
Abstract
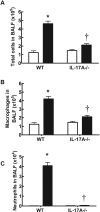
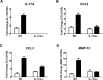
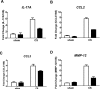
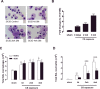
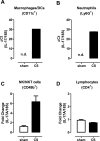
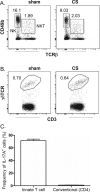
Cigarette smoke (CS) is the major cause of chronic obstructive pulmonary disease (COPD). Interleukin-17A (IL-17A) is a pivotal cytokine that regulates lung immunity and inflammation. The aim of the present study was to investigate how IL-17A regulates CS-induced lung inflammation in vivo. IL-17A knockout (KO) mice and neutralization of IL-17A in wild-type (WT) mice reduced macrophage and neutrophil recruitment and chemokine (C-C motif) ligand 2 (CCL2), CCL3 and matrix metalloproteinase (MMP)-12 mRNA expression in response to acute CS exposure. IL-17A expression was increased in non-obese diabetic (NOD) severe combined immunodeficiency SCID) mice with non-functional B- and T-cells over a 4-week CS exposure period, where macrophages accumulated to the same extent as in WT mice. Gene expression analysis by QPCR (quantitative real-time PCR) of isolated immune cell subsets detected increased levels of IL-17A transcript in macrophages, neutrophils and NK/NKT cells in the lungs of CS-exposed mice. In order to further explore the relative contribution of innate immune cellular sources, intracellular IL-17A staining was performed. In the present study, we demonstrate that CS exposure primes natural killer (NK), natural killer T (NKT) and γδ T-cells to produce more IL-17A protein and CS alone increased the frequency of IL17+ γδ T-cells in the lung, whereas IL-17A protein was not detected in macrophages and neutrophils. Our data suggest that activation of innate cellular sources of IL-17A is an essential mediator of macrophage accumulation in CS-exposed lungs. Targeting non-conventional T-cell sources of IL-17A may offer an alternative strategy to reduce pathogenic macrophages in COPD.
Keywords: chronic lung disease; chronic obstructive pulmonary disease (COPD); cigarette smoke; innate immunity; interleukin-17; macrophage.
© 2015 Authors; published by Portland Press Limited.
Figures


Similar articles
-
Genetic deletion of IL-17A reduces cigarette smoke-induced inflammation and alveolar type II cell apoptosis.Am J Physiol Lung Cell Mol Physiol. 2014 Jan;306(2):L132-43. doi: 10.1152/ajplung.00111.2013. Epub 2013 Oct 4. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24097560
-
Leptin modulates innate and adaptive immune cell recruitment after cigarette smoke exposure in mice.J Immunol. 2010 Jun 15;184(12):7169-77. doi: 10.4049/jimmunol.0900963. Epub 2010 May 19. J Immunol. 2010. PMID: 20488786
-
IL-17A and serum amyloid A are elevated in a cigarette smoke cessation model associated with the persistence of pigmented macrophages, neutrophils and activated NK cells.PLoS One. 2014 Nov 18;9(11):e113180. doi: 10.1371/journal.pone.0113180. eCollection 2014. PLoS One. 2014. PMID: 25405776 Free PMC article.
-
Cigarette Smoke-mediated Perturbations of the Immune Response: A New Therapeutic Approach with Natural Compounds.Endocr Metab Immune Disord Drug Targets. 2016;16(3):158-167. doi: 10.2174/1872214810666160927123447. Endocr Metab Immune Disord Drug Targets. 2016. PMID: 27697073 Review.
-
Th17 profile in COPD exacerbations.Int J Chron Obstruct Pulmon Dis. 2017 Jun 22;12:1857-1865. doi: 10.2147/COPD.S136592. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 28694696 Free PMC article. Review.
Cited by
-
B-Cell Activating Factor Secreted by Neutrophils Is a Critical Player in Lung Inflammation to Cigarette Smoke Exposure.Front Immunol. 2020 Jul 29;11:1622. doi: 10.3389/fimmu.2020.01622. eCollection 2020. Front Immunol. 2020. PMID: 32849550 Free PMC article.
-
Cigarette smoke depletes alveolar macrophages and delays clearance of Legionella pneumophila.Am J Physiol Lung Cell Mol Physiol. 2023 Mar 1;324(3):L373-L384. doi: 10.1152/ajplung.00268.2022. Epub 2023 Jan 31. Am J Physiol Lung Cell Mol Physiol. 2023. PMID: 36719079 Free PMC article.
-
Aminopeptidase N/CD13 Crosslinking Promotes the Activation and Membrane Expression of Integrin CD11b/CD18.Biomolecules. 2023 Oct 6;13(10):1488. doi: 10.3390/biom13101488. Biomolecules. 2023. PMID: 37892170 Free PMC article.
-
Prior cigarette smoke exposure does not affect acute post-stroke outcomes in mice.PLoS One. 2019 Mar 21;14(3):e0214246. doi: 10.1371/journal.pone.0214246. eCollection 2019. PLoS One. 2019. PMID: 30897180 Free PMC article.
-
Molecular and Cellular Mechanisms Responsible for Beneficial Effects of Mesenchymal Stem Cell-Derived Product "Exo-d-MAPPS" in Attenuation of Chronic Airway Inflammation.Anal Cell Pathol (Amst). 2020 Mar 20;2020:3153891. doi: 10.1155/2020/3153891. eCollection 2020. Anal Cell Pathol (Amst). 2020. PMID: 32257769 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

