Intracarotid Infusion of Mesenchymal Stem Cells in an Animal Model of Parkinson's Disease, Focusing on Cell Distribution and Neuroprotective and Behavioral Effects
- PMID: 26198165
- PMCID: PMC4542871
- DOI: 10.5966/sctm.2015-0023
Intracarotid Infusion of Mesenchymal Stem Cells in an Animal Model of Parkinson's Disease, Focusing on Cell Distribution and Neuroprotective and Behavioral Effects
Abstract
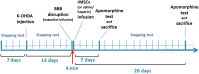
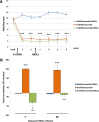
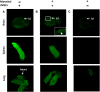
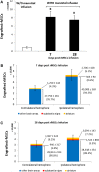
Mesenchymal stem cells (MSCs) have been proposed as a potential therapeutic tool for Parkinson's disease (PD) and systemic administration of these cells has been tested in preclinical and clinical studies. However, no information on survival and actual capacity of MSCs to reach the brain has been provided. In this study, we evaluated homing of intraarterially infused rat MSCs (rMSCs) in the brain of rats bearing a 6-hydroxydopamine (6-OHDA)-induced lesion of the nigrostriatal tract, to establish whether the toxin-induced damage is sufficient to grant MSC passage across the blood-brain barrier (BBB) or if a transient BBB disruption is necessary. The rMSC distribution in peripheral organs and the effects of cell infusion on neurodegenerative process and motor deficits were also investigated. rMSCs were infused 14 days after 6-OHDA injection. A hyperosmolar solution of mannitol was used to transiently permeabilize the BBB. Behavioral impairment was assessed by adjusting step test and response to apomorphine. Animals were sacrificed 7 and 28 days after cell infusion. Our work shows that appreciable delivery of rMSCs to the brain of 6-OHDA-lesioned animals can be obtained only after mannitol pretreatment. A notable percentage of infused cells accumulated in peripheral organs. Infusion of rMSCs did not modify the progression of 6-OHDA-induced damage or the motor impairment at the stepping test, but induced progressive normalization of the pathological response (contralateral turning) to apomorphine administration. These findings suggest that many aspects should be further investigated before considering any translation of MSC systemic administration into the clinical setting for PD treatment.
Significance: This study demonstrates that mesenchymal stem cells infused through the carotid artery do not efficiently cross the blood-brain barrier in rats with a Parkinson's disease-like degeneration of nigrostriatal neurons, unless a permeabilizing agent (e.g., mannitol) is used. The infusion did not reduce the neuronal damage and associated motor impairment, but abolished the motor abnormalities these animals typically show when challenged with a dopaminergic agonist. Therefore, although arterially infused mesenchymal stem cells did not show neurorestorative effects in this study's Parkinson's disease model, they appeared to normalize the pathological responsiveness of striatal neurons to dopaminergic stimulation. This capability should be further explored in future studies.
Keywords: Behavioral analyses; Intracarotid infusion; Mannitol; Mesenchymal stem cells; Neuroprotection; Parkinson’s disease.
©AlphaMed Press.
Figures


Similar articles
-
Transplantation of undifferentiated human mesenchymal stem cells protects against 6-hydroxydopamine neurotoxicity in the rat.Cell Transplant. 2010;19(2):203-17. doi: 10.3727/096368909X479839. Epub 2009 Nov 10. Cell Transplant. 2010. PMID: 19906332
-
Acupuncture prevents 6-hydroxydopamine-induced neuronal death in the nigrostriatal dopaminergic system in the rat Parkinson's disease model.Exp Neurol. 2003 Mar;180(1):93-8. doi: 10.1016/s0014-4886(02)00031-6. Exp Neurol. 2003. PMID: 12668152
-
GDNF-secreting mesenchymal stem cells provide localized neuroprotection in an inflammation-driven rat model of Parkinson's disease.Neuroscience. 2015 Sep 10;303:402-11. doi: 10.1016/j.neuroscience.2015.07.014. Epub 2015 Jul 10. Neuroscience. 2015. PMID: 26166730
-
Animal models of Parkinson's disease: an empirical comparison with the phenomenology of the disease in man.J Neural Transm (Vienna). 1996;103(8-9):987-1041. doi: 10.1007/BF01291788. J Neural Transm (Vienna). 1996. PMID: 9013391 Review.
-
Nanoformulation: A Useful Therapeutic Strategy for Improving Neuroprotection and the Neurorestorative Potential in Experimental Models of Parkinson's Disease.Int Rev Neurobiol. 2017;137:99-122. doi: 10.1016/bs.irn.2017.09.003. Epub 2017 Oct 20. Int Rev Neurobiol. 2017. PMID: 29132545 Review.
Cited by
-
Challenges and Controversies in Human Mesenchymal Stem Cell Therapy.Stem Cells Int. 2019 Apr 9;2019:9628536. doi: 10.1155/2019/9628536. eCollection 2019. Stem Cells Int. 2019. PMID: 31093291 Free PMC article. Review.
-
Therapeutic utility of mesenchymal stromal cell (MSC)-based approaches in chronic neurodegeneration: a glimpse into underlying mechanisms, current status, and prospects.Cell Mol Biol Lett. 2022 Jul 16;27(1):56. doi: 10.1186/s11658-022-00359-z. Cell Mol Biol Lett. 2022. PMID: 35842587 Free PMC article. Review.
-
Biodistribution of Mesenchymal Stromal Cells after Administration in Animal Models and Humans: A Systematic Review.J Clin Med. 2021 Jun 29;10(13):2925. doi: 10.3390/jcm10132925. J Clin Med. 2021. PMID: 34210026 Free PMC article. Review.
-
Impact of the Secretome of Human Mesenchymal Stem Cells on Brain Structure and Animal Behavior in a Rat Model of Parkinson's Disease.Stem Cells Transl Med. 2017 Feb;6(2):634-646. doi: 10.5966/sctm.2016-0071. Epub 2016 Sep 22. Stem Cells Transl Med. 2017. PMID: 28191785 Free PMC article.
-
Mesenchymal stromal cell biotherapy for Parkinson's disease premotor symptoms.Chin Neurosurg J. 2023 Oct 13;9(1):28. doi: 10.1186/s41016-023-00338-z. Chin Neurosurg J. 2023. PMID: 37833807 Free PMC article. Review.
References
-
- Defer GL, Geny C, Ricolfi F, et al. Long-term outcome of unilaterally transplanted parkinsonian patients. I. Clinical approach. Brain. 1996;119:41–50. - PubMed
-
- Freed CR, Breeze RE, Rosenberg NL, et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson’s disease. N Engl J Med. 1992;327:1549–1555. - PubMed
-
- Lindvall O, Brundin P, Widner H, et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science. 1990;247:574–577. - PubMed
-
- Madrazo I, León V, Torres C, et al. Transplantation of fetal substantia nigra and adrenal medulla to the caudate nucleus in two patients with Parkinson’s disease. N Engl J Med. 1988;318:51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

