Analysis of the interplay of protein biogenesis factors at the ribosome exit site reveals new role for NAC
- PMID: 26195668
- PMCID: PMC4508901
- DOI: 10.1083/jcb.201410086
Analysis of the interplay of protein biogenesis factors at the ribosome exit site reveals new role for NAC
Abstract
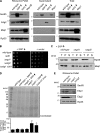
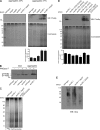
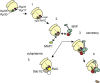
The ribosome exit site is a focal point for the interaction of protein-biogenesis factors that guide the fate of nascent polypeptides. These factors include chaperones such as NAC, N-terminal-modifying enzymes like Methionine aminopeptidase (MetAP), and the signal recognition particle (SRP), which targets secretory and membrane proteins to the ER. These factors potentially compete with one another in the short time-window when the nascent chain first emerges at the exit site, suggesting a need for regulation. Here, we show that MetAP contacts the ribosome at the universal adaptor site where it is adjacent to the α subunit of NAC. SRP is also known to contact the ribosome at this site. In the absence of NAC, MetAP and SRP antagonize each other, indicating a novel role for NAC in regulating the access of MetAP and SRP to the ribosome. NAC also functions in SRP-dependent targeting and helps to protect substrates from aggregation before translocation.
© 2015 Nyathi and Pool.
Figures




Comment in
-
Translation: competition at the ribosome exit site.Nat Rev Mol Cell Biol. 2015 Sep;16(9):516. doi: 10.1038/nrm4046. Epub 2015 Aug 12. Nat Rev Mol Cell Biol. 2015. PMID: 26265407 No abstract available.
Similar articles
-
The intrinsic ability of ribosomes to bind to endoplasmic reticulum membranes is regulated by signal recognition particle and nascent-polypeptide-associated complex.Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9435-9. doi: 10.1073/pnas.92.21.9435. Proc Natl Acad Sci U S A. 1995. PMID: 7568149 Free PMC article.
-
NAC functions as a modulator of SRP during the early steps of protein targeting to the endoplasmic reticulum.Mol Biol Cell. 2012 Aug;23(16):3027-40. doi: 10.1091/mbc.E12-02-0112. Epub 2012 Jun 27. Mol Biol Cell. 2012. PMID: 22740632 Free PMC article.
-
Cryo-EM Structures Reveal Relocalization of MetAP in the Presence of Other Protein Biogenesis Factors at the Ribosomal Tunnel Exit.J Mol Biol. 2019 Mar 29;431(7):1426-1439. doi: 10.1016/j.jmb.2019.02.002. Epub 2019 Feb 10. J Mol Biol. 2019. PMID: 30753870
-
SRP meets the ribosome.Nat Struct Mol Biol. 2004 Nov;11(11):1049-53. doi: 10.1038/nsmb853. Nat Struct Mol Biol. 2004. PMID: 15523481 Review.
-
Cotranslational sorting and processing of newly synthesized proteins in eukaryotes.Trends Biochem Sci. 2024 Feb;49(2):105-118. doi: 10.1016/j.tibs.2023.10.003. Epub 2023 Nov 1. Trends Biochem Sci. 2024. PMID: 37919225 Review.
Cited by
-
Multi-protein assemblies orchestrate co-translational enzymatic processing on the human ribosome.Nat Commun. 2024 Sep 3;15(1):7681. doi: 10.1038/s41467-024-51964-9. Nat Commun. 2024. PMID: 39227397 Free PMC article.
-
Role for ribosome-associated complex and stress-seventy subfamily B (RAC-Ssb) in integral membrane protein translation.J Biol Chem. 2017 Dec 1;292(48):19610-19627. doi: 10.1074/jbc.M117.813857. Epub 2017 Oct 2. J Biol Chem. 2017. PMID: 28972146 Free PMC article.
-
Structural basis of HypK regulating N-terminal acetylation by the NatA complex.Nat Commun. 2017 Jun 6;8:15726. doi: 10.1038/ncomms15726. Nat Commun. 2017. PMID: 28585574 Free PMC article.
-
Cotranslational signal-independent SRP preloading during membrane targeting.Nature. 2016 Aug 11;536(7615):224-8. doi: 10.1038/nature19309. Epub 2016 Aug 3. Nature. 2016. PMID: 27487213 Free PMC article.
-
Chaperone Interactions at the Ribosome.Cold Spring Harb Perspect Biol. 2019 Nov 1;11(11):a033977. doi: 10.1101/cshperspect.a033977. Cold Spring Harb Perspect Biol. 2019. PMID: 30833456 Free PMC article. Review.
References
-
- Becker T., Bhushan S., Jarasch A., Armache J.P., Funes S., Jossinet F., Gumbart J., Mielke T., Berninghausen O., Schulten K., et al. . 2009. Structure of monomeric yeast and mammalian Sec61 complexes interacting with the translating ribosome. Science. 326:1369–1373. 10.1126/science.1178535 - DOI - PMC - PubMed
-
- Boissel J.P., Kasper T.J., and Bunn H.F.. 1988. Cotranslational amino-terminal processing of cytosolic proteins. Cell-free expression of site-directed mutants of human hemoglobin. J. Biol. Chem. 263:8443–8449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

