Bone Marrow Gene Therapy for HIV/AIDS
- PMID: 26193303
- PMCID: PMC4517133
- DOI: 10.3390/v7072804
Bone Marrow Gene Therapy for HIV/AIDS
Abstract
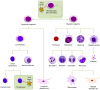
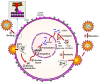
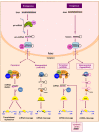
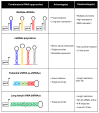
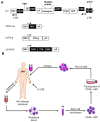
Bone marrow gene therapy remains an attractive option for treating chronic immunological diseases, including acquired immunodeficiency syndrome (AIDS) caused by human immunodeficiency virus (HIV). This technology combines the differentiation and expansion capacity of hematopoietic stem cells (HSCs) with long-term expression of therapeutic transgenes using integrating vectors. In this review we summarize the potential of bone marrow gene therapy for the treatment of HIV/AIDS. A broad range of antiviral strategies are discussed, with a particular focus on RNA-based therapies. The idea is to develop a durable gene therapy that lasts the life span of the infected individual, thus contrasting with daily drug regimens to suppress the virus. Different approaches have been proposed to target either the virus or cellular genes encoding co-factors that support virus replication. Some of these therapies have been tested in clinical trials, providing proof of principle that gene therapy is a safe option for treating HIV/AIDS. In this review several topics are discussed, ranging from the selection of the antiviral molecule and the viral target to the optimal vector system for gene delivery and the setup of appropriate preclinical test systems. The molecular mechanisms used to formulate a cure for HIV infection are described, including the latest antiviral strategies and their therapeutic applications. Finally, a potent combination of anti-HIV genes based on our own research program is described.
Keywords: HIV-1; RNAi; antiviral; bone marrow; gene therapy; hematopoietic stem cell (HSC); lentiviral vector; virus.
Figures
Similar articles
-
Therapeutic genes for anti-HIV/AIDS gene therapy.Curr Pharm Biotechnol. 2013;14(5):488-500. doi: 10.2174/138920101405131111104009. Curr Pharm Biotechnol. 2013. PMID: 22429132 Review.
-
Hematopoietic stem cell-based gene therapy for acquired immunodeficiency syndrome: efficient transduction and expression of RevM10 in myeloid cells in vivo and in vitro.Blood. 1997 Apr 1;89(7):2283-90. Blood. 1997. PMID: 9116270
-
Protection of stem cell-derived lymphocytes in a primate AIDS gene therapy model after in vivo selection.PLoS One. 2009 Nov 2;4(11):e7693. doi: 10.1371/journal.pone.0007693. PLoS One. 2009. PMID: 19888329 Free PMC article.
-
Recent advances in RNAi-based strategies for therapy and prevention of HIV-1/AIDS.Adv Drug Deliv Rev. 2016 Aug 1;103:174-186. doi: 10.1016/j.addr.2016.03.005. Epub 2016 Mar 21. Adv Drug Deliv Rev. 2016. PMID: 27013255 Free PMC article. Review.
-
Optimized lentiviral vectors for HIV gene therapy: multiplexed expression of small RNAs and inclusion of MGMT(P140K) drug resistance gene.Mol Ther. 2014 May;22(5):952-63. doi: 10.1038/mt.2014.32. Epub 2014 Feb 28. Mol Ther. 2014. PMID: 24576853 Free PMC article.
Cited by
-
Recent advances in gene therapy: genetic bullets to the root of the problem.Clin Exp Med. 2023 Aug;23(4):1107-1121. doi: 10.1007/s10238-022-00925-x. Epub 2022 Oct 25. Clin Exp Med. 2023. PMID: 36284069 Review.
-
Spotlight on the impact of viral infections on Hematopoietic Stem Cells (HSCs) with a focus on COVID-19 effects.Cell Commun Signal. 2023 May 8;21(1):103. doi: 10.1186/s12964-023-01122-3. Cell Commun Signal. 2023. PMID: 37158893 Free PMC article. Review.
-
Increased Efficiency for Biallelic Mutations of the CCR5 Gene by CRISPR-Cas9 Using Multiple Guide RNAs As a Novel Therapeutic Option for Human Immunodeficiency Virus.CRISPR J. 2021 Feb;4(1):92-103. doi: 10.1089/crispr.2020.0019. CRISPR J. 2021. PMID: 33616448 Free PMC article.
-
Subcellular Localization of HIV-1 gag-pol mRNAs Regulates Sites of Virion Assembly.J Virol. 2017 Feb 28;91(6):e02315-16. doi: 10.1128/JVI.02315-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28053097 Free PMC article.
-
Directed evolution of a recombinase that excises the provirus of most HIV-1 primary isolates with high specificity.Nat Biotechnol. 2016 Apr;34(4):401-9. doi: 10.1038/nbt.3467. Epub 2016 Feb 22. Nat Biotechnol. 2016. PMID: 26900663
References
-
- UNAIDS . Reports on the Global AIDS Epidemic. UNAIDS; Geneva, Switzerland: 2013.
-
- Gray G.E., Allen M., Moodie Z., Churchyard G., Bekker L.G., Nchabeleng M., Mlisana K., Metch B., De B.G., Latka M.H., et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: A double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect. Dis. 2011;11:507–515. doi: 10.1016/S1473-3099(11)70098-6. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

