Chronic treatment with varenicline changes expression of four nAChR binding sites in mice
- PMID: 26192545
- PMCID: PMC4655142
- DOI: 10.1016/j.neuropharm.2015.07.019
Chronic treatment with varenicline changes expression of four nAChR binding sites in mice
Abstract
Introduction: Chronic treatment with nicotine is known to increase the α4β2-nAChR sites in brain, to decrease α6β2-nAChR sites and to have minimal effect on α3β4-and α7-nAChR populations. Varenicline is now used as a smoking cessation treatment, with and without continued smoking or nicotine replacement therapy. Varenicline, like nicotine, upregulates the α4β2-nAChR sites; however, it is not known whether varenicline treatment changes expression of the other nAChR subtypes.
Methods: Using a mouse model, chronic treatments (10 days) with varenicline (0.12 mg/kg/h) and/or nicotine (1 mg/kg/hr), alone or in combination, were compared for plasma and brain levels of drugs, tolerance to subsequent acute nicotine and expression of four subtypes of nAChR using autoradiography.
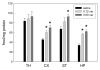
Results: The upregulation of α4β2-nAChR sites elicited by chronic varenicline was very similar to that elicited by chronic nicotine. Treatment with both drugs somewhat increased up-regulation, indicating that these doses were not quite at maximum effect. Similar down-regulation was seen for α6β2-nAChR sites. Varenicline significantly increased both α3β4-and α7-nAChR sites while nicotine had less effect on these sites. The drug combination was similar to varenicline alone for α3β4-nAChR sites, while for α7 sites the drug combination was less effective than varenicline alone. Varenicline had small but significant effects on tolerance to acute nicotine.
Conclusions: Effects of varenicline in vivo may not be limited to the α4β2*-nAChR subtype. In addition, smoking cessation treatment with varenicline may not allow receptor numbers to be restored to baseline and may, in addition, change expression of other receptor subtypes.
Keywords: Chronic treatment; Cytisine (PubChem CID: 10235); Epibatidine (PubChem CID: 3073763); Nicotine; Nicotine (PubChem CID: 942); Nicotinic receptors; Varenicline; Varenicline (PubChen CID: 5310966).
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
The contribution of α4β2 and non-α4β2 nicotinic acetylcholine receptors to the discriminative stimulus effects of nicotine and varenicline in mice.Psychopharmacology (Berl). 2017 Mar;234(5):781-792. doi: 10.1007/s00213-016-4514-4. Epub 2016 Dec 27. Psychopharmacology (Berl). 2017. PMID: 28028600 Free PMC article.
-
The contribution of agonist and antagonist activities of α4β2* nAChR ligands to smoking cessation efficacy: a quantitative analysis of literature data.Psychopharmacology (Berl). 2018 Sep;235(9):2479-2505. doi: 10.1007/s00213-018-4921-9. Epub 2018 Jul 7. Psychopharmacology (Berl). 2018. PMID: 29980822 Review.
-
Genetic deletion of the adenosine A(2A) receptor prevents nicotine-induced upregulation of α7, but not α4β2* nicotinic acetylcholine receptor binding in the brain.Neuropharmacology. 2013 Aug;71:228-36. doi: 10.1016/j.neuropharm.2013.03.023. Epub 2013 Apr 11. Neuropharmacology. 2013. PMID: 23583933
-
CC4, a dimer of cytisine, is a selective partial agonist at α4β2/α6β2 nAChR with improved selectivity for tobacco smoking cessation.Br J Pharmacol. 2013 Feb;168(4):835-49. doi: 10.1111/j.1476-5381.2012.02204.x. Br J Pharmacol. 2013. PMID: 22957729 Free PMC article.
-
Role for the nicotinic cholinergic system in movement disorders; therapeutic implications.Pharmacol Ther. 2014 Oct;144(1):50-9. doi: 10.1016/j.pharmthera.2014.05.004. Epub 2014 May 14. Pharmacol Ther. 2014. PMID: 24836728 Free PMC article. Review.
Cited by
-
Varenicline Targets the Reinforcing-Enhancing Effect of Nicotine on Its Associated Salient Cue During Nicotine Self-administration in the Rat.Front Behav Neurosci. 2019 Jul 17;13:159. doi: 10.3389/fnbeh.2019.00159. eCollection 2019. Front Behav Neurosci. 2019. PMID: 31379531 Free PMC article.
-
Discovery and development of varenicline for smoking cessation.Expert Opin Drug Discov. 2018 Jul;13(7):671-683. doi: 10.1080/17460441.2018.1458090. Epub 2018 Mar 28. Expert Opin Drug Discov. 2018. PMID: 29587555 Free PMC article. Review.
-
A Combination of Naltrexone + Varenicline Retards the Expression of a Genetic Predisposition Toward High Alcohol Drinking.Alcohol Clin Exp Res. 2017 Mar;41(3):644-652. doi: 10.1111/acer.13326. Epub 2017 Feb 9. Alcohol Clin Exp Res. 2017. PMID: 28055135 Free PMC article.
-
Neural circuits and nicotinic acetylcholine receptors mediate the cholinergic regulation of midbrain dopaminergic neurons and nicotine dependence.Acta Pharmacol Sin. 2020 Jan;41(1):1-9. doi: 10.1038/s41401-019-0299-4. Epub 2019 Sep 25. Acta Pharmacol Sin. 2020. PMID: 31554960 Free PMC article. Review.
-
Organelle-specific single-molecule imaging of α4β2 nicotinic receptors reveals the effect of nicotine on receptor assembly and cell-surface trafficking.J Biol Chem. 2017 Dec 22;292(51):21159-21169. doi: 10.1074/jbc.M117.801431. Epub 2017 Oct 26. J Biol Chem. 2017. PMID: 29074617 Free PMC article.
References
-
- Al-Haj A, Alawi M, Arafat T, Hourani MK. Method Development, validation and bioequivalence in human plasma by liquid chromatography tandem mass spectrometry. J. Chromatogr B Analyt. Technol. Biomed. Life Sci. 2013;931:134–139. - PubMed
-
- Benwell ME, Balfour DJ, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J Neurochem. 1988;50(4):1243–1247. - PubMed
-
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48(10):3474–3477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

