Clinical Translation of Cell Therapy, Tissue Engineering, and Regenerative Medicine Product in Malaysia and Its Regulatory Policy
- PMID: 26192075
- PMCID: PMC4684660
- DOI: 10.1089/ten.TEA.2014.0521
Clinical Translation of Cell Therapy, Tissue Engineering, and Regenerative Medicine Product in Malaysia and Its Regulatory Policy
Abstract
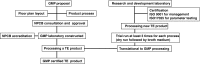
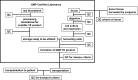
With the worldwide growth of cell and tissue therapy (CTT) in treating diseases, the need of a standardized regulatory policy is of paramount concern. Research in CTT in Malaysia has reached stages of clinical trials and commercialization. In Malaysia, the regulation of CTT is under the purview of the National Pharmaceutical Control Bureau (NPCB), Ministry of Health (MOH). NPCB is given the task of regulating CTT, under a new Cell and Gene Therapy Products framework, and the guidelines are currently being formulated. Apart from the laboratory accreditation, researchers are advised to follow Guidelines for Stem Cell Research and Therapy from the Medical Development Division, MOH, published in 2009.
Figures

Similar articles
-
Regulation of Regenerative Medicine Products.Adv Exp Med Biol. 2018;1098:189-198. doi: 10.1007/978-3-319-97421-7_10. Adv Exp Med Biol. 2018. PMID: 30238372 Review.
-
Regulation of Cell- and Tissue-Based Therapeutic Products in Singapore.Tissue Eng Part A. 2015 Dec;21(23-24):2802-5. doi: 10.1089/ten.TEA.2014.0712. Epub 2015 Jul 23. Tissue Eng Part A. 2015. PMID: 26096855
-
Regulation Policy on Cell- and Tissue-Based Therapy Products in Korea.Tissue Eng Part A. 2015 Dec;21(23-24):2791-6. doi: 10.1089/ten.TEA.2014.0429. Epub 2015 Jun 1. Tissue Eng Part A. 2015. PMID: 25759938
-
Premarket Regulation of Tissue Engineered Medical Products in China.Tissue Eng Part A. 2015 Dec;21(23-24):2806-11. doi: 10.1089/ten.TEA.2015.0423. Tissue Eng Part A. 2015. PMID: 26529310
-
From in vitro to in situ tissue engineering.Ann Biomed Eng. 2014 Jul;42(7):1537-45. doi: 10.1007/s10439-014-1022-8. Epub 2014 May 9. Ann Biomed Eng. 2014. PMID: 24809723 Review.
Cited by
-
Current standards and ethical landscape of engineered tissues-3D bioprinting perspective.J Tissue Eng. 2021 Jul 29;12:20417314211027677. doi: 10.1177/20417314211027677. eCollection 2021 Jan-Dec. J Tissue Eng. 2021. PMID: 34377431 Free PMC article. Review.
-
Natural Killer Cell-Derived Extracellular Vesicles as a Promising Immunotherapeutic Strategy for Cancer: A Systematic Review.Int J Mol Sci. 2023 Feb 16;24(4):4026. doi: 10.3390/ijms24044026. Int J Mol Sci. 2023. PMID: 36835438 Free PMC article. Review.
-
Application of stem cells in tissue engineering for defense medicine.Mil Med Res. 2018 Feb 26;5(1):7. doi: 10.1186/s40779-018-0154-9. Mil Med Res. 2018. PMID: 29502528 Free PMC article. Review.
-
Prospect of Metal Ceramic (Titanium-Wollastonite) Composite as Permanent Bone Implants: A Narrative Review.Materials (Basel). 2021 Jan 7;14(2):277. doi: 10.3390/ma14020277. Materials (Basel). 2021. PMID: 33430455 Free PMC article. Review.
-
Is Malaysia Ready for Human Gene Editing: A Regulatory, Biosafety and Biosecurity Perspective.Front Bioeng Biotechnol. 2021 Mar 11;9:649203. doi: 10.3389/fbioe.2021.649203. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33777918 Free PMC article. Review.
References
-
- Mason C., McCall M.J., Culme-Seymour E.J., Suthasan S., Edwards-Parton S., Bonfiglio G.A., and Reeve B.C. The global cell therapy industry continues to rise during the second and third quarters of 2012. Cell Stem Cell 11, 735, 2012 - PubMed
-
- Martin I., Baldomero H., Bocelli-Tyndall C., Passweg J., Saris D., and Tyndall A. The survey on cellular and engineered tissue therapies in Europe in 2010. Tissue Eng Part A 18, 2268, 2012 - PubMed
-
- Hellman K.B. Biomedical applications of tissue engineering technology: regulatory issues. Tissue Eng 1, 203, 1995 - PubMed
-
- Brévignon-Dodin L. Regulatory enablers and regulatory challenges for the development of tissue-engineered products in the EU. Biomed Mater Eng 20, 121, 2010 - PubMed
-
- Caunday O., Bensoussan D., Decot V., Bordigoni P., and Stoltz J.F. Regulatory aspects of cellular therapy product in Europe: JACIE accreditation in a processing facility. Biomed Mater Eng 19, 373, 2009 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

