Evaluation in usual practice of the bevacizumab-FOLFIRI combination for the first-line treatment of patients with unresectable metastatic colorectal cancer treated in 2006: focus on resected patients and oncogeriatrics: AVASTIN OUEST cohort of the Observatory of Cancer of the Brittany and Pays de la Loire Areas (Observatoire dédié au Cancer Bretagne / Pays de la Loire)
- PMID: 26190928
- PMCID: PMC4496868
- DOI: 10.1007/s10269-014-2391-1
Evaluation in usual practice of the bevacizumab-FOLFIRI combination for the first-line treatment of patients with unresectable metastatic colorectal cancer treated in 2006: focus on resected patients and oncogeriatrics: AVASTIN OUEST cohort of the Observatory of Cancer of the Brittany and Pays de la Loire Areas (Observatoire dédié au Cancer Bretagne / Pays de la Loire)
Abstract
Background: In 2006, bevacizumab, a targeted therapy agent was combined with FOLFIRI for the firstline treatment of patients with unresectable metastatic colorectal cancer.
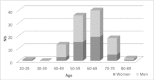
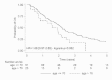
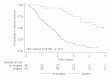
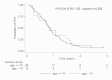
Methods/results: A study on a homogenous series of 111 patients from the Brittany and Pays de la Loire areas who received bevacizumab-FOLFIRI as first-line treatment in 2006 showed the following results: 51 responses, 29 stabilisations, 21 progressions and 10 cases of toxicity prior to assessment. Median overall survival (OS) was 25.1 months and median progression-free survival was 10.2 months. Surgery secondary to treatment tripled median OS which reached 59.2 months in resected patients versus 18.8 months in unresected patients. Comparison of patients aged more or less than 70 years showed no differences in terms of benefits or risks.
Conclusion: Bevacizumab-FOLFIRI could be administered as part of a routine care protocol to elderly patients previously evaluated by a geriatric assessment and validated by a multidisciplinary staff.
En 2006, bevacizumab-FOLFIRI représente la thérapie ciblée administrable dès la première ligne chez les patients porteurs d’un cancer colorectal métastatique non opérable. Une série homogène de 111 patients colligés en région Bretagne et Pays de la Loire ayant reçu du bevacizumab- FOLFIRI en première ligne en 2006 révèle les résultats suivants: 51 réponses, 29 stabilités, 21 progressions et 10 toxicités avant évaluation. La médiane de survie globale (OS) est de 25,1 mois et la médiane de survie sans progression (PFS) de 10,2 mois. Dans le cas d’une chirurgie secondaire, l’OS médian triple de 18,8 mois chez les patients non réséqués versus 59,2 mois ceux réséqués. En comparant les sujets âgés de plus et de moins de 70 ans, aucune différence n’a été mise en évidence en termes de bénéfice ou de risque. Bevacizumab-FOLFIRI pourrait être administré en pratique courante chez les personnes âgées sous couvert d’une évaluation gériatrique et d’une approche multidisciplinaire.
Keywords: Bevacizumab; Cohort; Daily practice; Elderly patients; Liver surgical resection; Metastatic colorectal cancer.
Figures
Similar articles
-
Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): a multicentre, open-label, randomised, non-inferiority, phase 3 trial.Lancet Oncol. 2018 May;19(5):660-671. doi: 10.1016/S1470-2045(18)30140-2. Epub 2018 Mar 16. Lancet Oncol. 2018. PMID: 29555258 Clinical Trial.
-
Protocol of the EFFORT study: a prospective study of FOLFIRI plus aflibercept as second-line treatment after progression on FOLFOXIRI plus bevacizumab or during maintenance treatment in patients with unresectable/metastatic colorectal cancer.BMC Cancer. 2020 Nov 17;20(1):1116. doi: 10.1186/s12885-020-07576-9. BMC Cancer. 2020. PMID: 33203393 Free PMC article.
-
First-line systemic treatment strategies in patients with initially unresectable colorectal cancer liver metastases (CAIRO5): an open-label, multicentre, randomised, controlled, phase 3 study from the Dutch Colorectal Cancer Group.Lancet Oncol. 2023 Jul;24(7):757-771. doi: 10.1016/S1470-2045(23)00219-X. Epub 2023 Jun 14. Lancet Oncol. 2023. PMID: 37329889 Clinical Trial.
-
Chemotherapy of metastatic colorectal cancer.Prescrire Int. 2010 Oct;19(109):219-24. Prescrire Int. 2010. PMID: 21180382 Review.
-
FOLFIRI-bevacizumab as first-line chemotherapy in 3500 patients with advanced colorectal cancer: a pooled analysis of 29 published trials.Clin Colorectal Cancer. 2013 Sep;12(3):145-51. doi: 10.1016/j.clcc.2013.04.006. Epub 2013 Jun 10. Clin Colorectal Cancer. 2013. PMID: 23763824 Review.
References
-
- Bokemeyer C, Van Cutsem E, Rougier P, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48(10):1466–1475. doi: 10.1016/j.ejca.2012.02.057. - DOI - PubMed
-
- Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697–4705. doi: 10.1200/JCO.2009.27.4860. - DOI - PubMed
-
- Pectasides D, Papaxoinis G, Kalogeras K, et al. XELIRI- Bevacizumab versus FOLFIRI-Bevacizumab as first line treatment in patients with metastatic colorectal cancer: a Hellenic Cooperative Oncology group phase III Trial with collateral biomarker analysis. BMC Cancer. 2012;12:271. doi: 10.1186/1471-2407-12-271. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
