Controlling drug delivery kinetics from mesoporous titania thin films by pore size and surface energy
- PMID: 26185444
- PMCID: PMC4501225
- DOI: 10.2147/IJN.S83005
Controlling drug delivery kinetics from mesoporous titania thin films by pore size and surface energy
Abstract
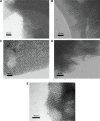
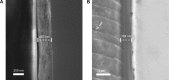
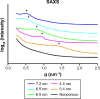
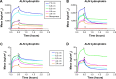
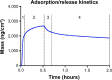
The osseointegration capacity of bone-anchoring implants can be improved by the use of drugs that are administrated by an inbuilt drug delivery system. However, to attain superior control of drug delivery and to have the ability to administer drugs of varying size, including proteins, further material development of drug carriers is needed. Mesoporous materials have shown great potential in drug delivery applications to provide and maintain a drug concentration within the therapeutic window for the desired period of time. Moreover, drug delivery from coatings consisting of mesoporous titania has shown to be promising to improve healing of bone-anchoring implants. Here we report on how the delivery of an osteoporosis drug, alendronate, can be controlled by altering pore size and surface energy of mesoporous titania thin films. The pore size was varied from 3.4 nm to 7.2 nm by the use of different structure-directing templates and addition of a swelling agent. The surface energy was also altered by grafting dimethylsilane to the pore walls. The drug uptake and release profiles were monitored in situ using quartz crystal microbalance with dissipation (QCM-D) and it was shown that both pore size and surface energy had a profound effect on both the adsorption and release kinetics of alendronate. The QCM-D data provided evidence that the drug delivery from mesoporous titania films is controlled by a binding-diffusion mechanism. The yielded knowledge of release kinetics is crucial in order to improve the in vivo tissue response associated to therapeutic treatments.
Keywords: QCM-D; alendronate; controlled drug delivery; mesoporous titania; release kinetics.
Figures

Similar articles
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Far Posterior Approach for Rib Fracture Fixation: Surgical Technique and Tips.JBJS Essent Surg Tech. 2024 Dec 6;14(4):e23.00094. doi: 10.2106/JBJS.ST.23.00094. eCollection 2024 Oct-Dec. JBJS Essent Surg Tech. 2024. PMID: 39650795 Free PMC article.
-
Interventions to reduce harm from continued tobacco use.Cochrane Database Syst Rev. 2016 Oct 13;10(10):CD005231. doi: 10.1002/14651858.CD005231.pub3. Cochrane Database Syst Rev. 2016. PMID: 27734465 Free PMC article. Review.
Cited by
-
pH-Responsive Cobalt(II)-Coordinated Assembly Containing Quercetin for Antimicrobial Applications.Molecules. 2023 Jul 22;28(14):5581. doi: 10.3390/molecules28145581. Molecules. 2023. PMID: 37513453 Free PMC article.
-
Functionalized Mesoporous Thin Films for Biotechnology.Micromachines (Basel). 2021 Jun 24;12(7):740. doi: 10.3390/mi12070740. Micromachines (Basel). 2021. PMID: 34202530 Free PMC article. Review.
-
The effect of magnesium on early osseointegration in osteoporotic bone: a histological and gene expression investigation.Osteoporos Int. 2017 Jul;28(7):2195-2205. doi: 10.1007/s00198-017-4004-5. Epub 2017 Mar 27. Osteoporos Int. 2017. PMID: 28349251 Free PMC article.
-
Studying the Drug Delivery Kinetics of a Nanoporous Matrix Using a MIP-Based Thermal Sensing Platform.Polymers (Basel). 2017 Oct 28;9(11):560. doi: 10.3390/polym9110560. Polymers (Basel). 2017. PMID: 30965864 Free PMC article.
-
Osseointegration effects of local release of strontium ranelate from implant surfaces in rats.J Mater Sci Mater Med. 2019 Oct 12;30(10):116. doi: 10.1007/s10856-019-6314-y. J Mater Sci Mater Med. 2019. PMID: 31606798 Free PMC article.
References
-
- Puleo DA, Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999;20(23–24):2311–2321. - PubMed
-
- Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone. 1996;18(2):75–85. - PubMed
-
- Kimmel DB. Mechanism of action, pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. J Dent Res. 2007;86(11):1022–1033. - PubMed
-
- Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19(6):733–759. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

