Atherosclerosis following renal injury is ameliorated by pioglitazone and losartan via macrophage phenotype
- PMID: 26184694
- PMCID: PMC4850906
- DOI: 10.1016/j.atherosclerosis.2015.06.055
Atherosclerosis following renal injury is ameliorated by pioglitazone and losartan via macrophage phenotype
Abstract
Objective: Chronic kidney disease (CKD) amplifies atherosclerosis, which involves renin-angiotensin system (RAS) regulation of macrophages. RAS influences peroxisome proliferator-activated receptor-γ (PPARγ), a modulator of atherogenic functions of macrophages, however, little is known about its effects in CKD. We examined the impact of combined therapy with a PPARγ agonist and angiotensin receptor blocker on atherogenesis in a murine uninephrectomy model.
Methods: Apolipoprotein E knockout mice underwent uninephrectomy (UNx) and treatment with pioglitazone (UNx + Pio), losartan (UNx + Los), or both (UNx + Pio/Los) for 10 weeks. Extent and characteristics of atherosclerotic lesions and macrophage phenotypes were assessed; RAW264.7 and primary peritoneal mouse cells were used to examine pioglitazone and losartan effects on macrophage phenotype and inflammatory response.
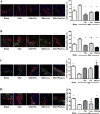
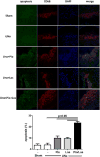
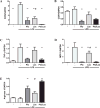
Results: UNx significantly increased atherosclerosis. Pioglitazone and losartan each significantly reduced the atherosclerotic burden by 29.6% and 33.5%, respectively; although the benefit was dramatically augmented by combination treatment which lessened atherosclerosis by 55.7%. Assessment of plaques revealed significantly greater macrophage area in UNx + Pio/Los (80.7 ± 11.4% vs. 50.3 ± 4.2% in UNx + Pio and 57.2 ± 6.5% in UNx + Los) with more apoptotic cells. The expanded macrophage-rich lesions of UNx + Pio/Los had more alternatively activated, Ym-1 and arginine 1-positive M2 phenotypes (Ym-1: 33.6 ± 8.2%, p < 0.05 vs. 12.0 ± 1.1% in UNx; arginase 1: 27.8 ± 0.9%, p < 0.05 vs. 11.8 ± 1.3% in UNx). In vitro, pioglitazone alone and together with losartan was more effective than losartan alone in dampening lipopolysaccharide-induced cytokine production, suppressing M1 phenotypic change while enhancing M2 phenotypic change.
Conclusion: Combination of pioglitazone and losartan is more effective in reducing renal injury-induced atherosclerosis than either treatment alone. This benefit reflects mitigation in macrophage cytokine production, enhanced apoptosis, and a shift toward an anti-inflammatory phenotype.
Keywords: Atherosclerosis; Chronic kidney disease; Losartan; Macrophage phenotype; PPARγ; Pioglitazone.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Figures

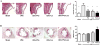
Similar articles
-
Macrophage polarization by angiotensin II-type 1 receptor aggravates renal injury-acceleration of atherosclerosis.Arterioscler Thromb Vasc Biol. 2011 Dec;31(12):2856-64. doi: 10.1161/ATVBAHA.111.237198. Epub 2011 Oct 6. Arterioscler Thromb Vasc Biol. 2011. PMID: 21979434 Free PMC article.
-
Pioglitazone-Incorporated Nanoparticles Prevent Plaque Destabilization and Rupture by Regulating Monocyte/Macrophage Differentiation in ApoE-/- Mice.Arterioscler Thromb Vasc Biol. 2016 Mar;36(3):491-500. doi: 10.1161/ATVBAHA.115.307057. Epub 2016 Jan 28. Arterioscler Thromb Vasc Biol. 2016. PMID: 26821947
-
Comparison of angiotensin-(1-7), losartan and their combination on atherosclerotic plaque formation in apolipoprotein E knockout mice.Atherosclerosis. 2015 Jun;240(2):544-9. doi: 10.1016/j.atherosclerosis.2015.02.055. Epub 2015 Mar 3. Atherosclerosis. 2015. PMID: 25957120
-
Effects of PPARγ on hypertension, atherosclerosis, and chronic kidney disease.Endocr J. 2010;57(10):847-52. doi: 10.1507/endocrj.k10e-281. Epub 2010 Sep 28. Endocr J. 2010. PMID: 20890053 Review.
-
[Roles of PPARgamma in preventing the development of atherosclerosis in LDL receptor null mice].Nihon Rinsho. 2010 Feb;68(2):229-34. Nihon Rinsho. 2010. PMID: 20158089 Review. Japanese.
Cited by
-
Cucumis sativus Aqueous Fraction Inhibits Angiotensin II-Induced Inflammation and Oxidative Stress In Vitro.Nutrients. 2018 Feb 28;10(3):276. doi: 10.3390/nu10030276. Nutrients. 2018. PMID: 29495578 Free PMC article.
-
Assessment of the Relationship between Carotid Intima-Media Thickening and Early-Stage Diabetic Kidney Disease Coupled with Helicobacter pylori Infection.Dis Markers. 2018 Apr 16;2018:3793768. doi: 10.1155/2018/3793768. eCollection 2018. Dis Markers. 2018. PMID: 29849820 Free PMC article.
-
Immunomodulatory Activity of the Most Commonly Used Antihypertensive Drugs-Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers.Int J Mol Sci. 2022 Feb 4;23(3):1772. doi: 10.3390/ijms23031772. Int J Mol Sci. 2022. PMID: 35163696 Free PMC article. Review.
-
Pioglitazone treatment mitigates cardiovascular bioprosthetic degeneration in a chronic kidney disease model.Front Pharmacol. 2024 Aug 8;15:1412169. doi: 10.3389/fphar.2024.1412169. eCollection 2024. Front Pharmacol. 2024. PMID: 39175545 Free PMC article.
-
A Nomogram for Identifying Subclinical Atherosclerosis in Chronic Kidney Disease.Clin Interv Aging. 2021 Jul 8;16:1303-1313. doi: 10.2147/CIA.S312129. eCollection 2021. Clin Interv Aging. 2021. PMID: 34267510 Free PMC article.
References
-
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. - PubMed
-
- Klausen KP, Scharling H, Jensen JS. Very low level of microalbuminuria is associated with increased risk of death in subjects with cardiovascular or cerebrovascular diseases. J Intern Med. 2006;260:231–237. - PubMed
-
- Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. - PubMed
-
- Levey AS, de Jong PE, Coresh J, Nahas MEl, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO controversies conference report. Kidney Int. 2011;80:17–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

