Eosinophil-Derived Neurotoxin (EDN/RNase 2) and the Mouse Eosinophil-Associated RNases (mEars): Expanding Roles in Promoting Host Defense
- PMID: 26184157
- PMCID: PMC4519907
- DOI: 10.3390/ijms160715442
Eosinophil-Derived Neurotoxin (EDN/RNase 2) and the Mouse Eosinophil-Associated RNases (mEars): Expanding Roles in Promoting Host Defense
Abstract
The eosinophil-derived neurotoxin (EDN/RNase2) and its divergent orthologs, the mouse eosinophil-associated RNases (mEars), are prominent secretory proteins of eosinophilic leukocytes and are all members of the larger family of RNase A-type ribonucleases. While EDN has broad antiviral activity, targeting RNA viruses via mechanisms that may require enzymatic activity, more recent studies have elucidated how these RNases may generate host defense via roles in promoting leukocyte activation, maturation, and chemotaxis. This review provides an update on recent discoveries, and highlights the versatility of this family in promoting innate immunity.
Keywords: chemoattractant; evolution; inflammation; leukocyte.
Figures

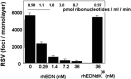


Similar articles
-
Human ribonuclease A superfamily members, eosinophil-derived neurotoxin and pancreatic ribonuclease, induce dendritic cell maturation and activation.J Immunol. 2004 Nov 15;173(10):6134-42. doi: 10.4049/jimmunol.173.10.6134. J Immunol. 2004. PMID: 15528350 Free PMC article.
-
Eosinophil-derived neurotoxin / RNase 2: connecting the past, the present and the future.Curr Pharm Biotechnol. 2008 Jun;9(3):135-40. doi: 10.2174/138920108784567236. Curr Pharm Biotechnol. 2008. PMID: 18673278 Free PMC article. Review.
-
An insertion in loop L7 of human eosinophil-derived neurotoxin is crucial for its antiviral activity.J Cell Biochem. 2012 Oct;113(10):3104-12. doi: 10.1002/jcb.24187. J Cell Biochem. 2012. PMID: 22581709
-
Eosinophils, ribonucleases and host defense: solving the puzzle.Immunol Res. 1999;20(3):261-74. doi: 10.1007/BF02790409. Immunol Res. 1999. PMID: 10741866 Review.
-
Eosinophil-derived neurotoxin (EDN), an antimicrobial protein with chemotactic activities for dendritic cells.Blood. 2003 Nov 1;102(9):3396-403. doi: 10.1182/blood-2003-01-0151. Epub 2003 Jul 10. Blood. 2003. PMID: 12855582
Cited by
-
Serum Levels of Eosinophil-Derived Neurotoxin, Platelet-Activating Factor and Vascular Endothelial Growth Factor in Adult Patients with Atopic Dermatitis-A Pilot Study.Biomedicines. 2022 Dec 1;10(12):3109. doi: 10.3390/biomedicines10123109. Biomedicines. 2022. PMID: 36551865 Free PMC article.
-
Human Antimicrobial RNases Inhibit Intracellular Bacterial Growth and Induce Autophagy in Mycobacteria-Infected Macrophages.Front Immunol. 2019 Jul 2;10:1500. doi: 10.3389/fimmu.2019.01500. eCollection 2019. Front Immunol. 2019. PMID: 31312205 Free PMC article.
-
Assessment of Lung Eosinophils In Situ Using Immunohistological Staining.Methods Mol Biol. 2021;2223:237-266. doi: 10.1007/978-1-0716-1001-5_17. Methods Mol Biol. 2021. PMID: 33226599 Free PMC article.
-
Conformational exchange divergence along the evolutionary pathway of eosinophil-associated ribonucleases.Structure. 2023 Mar 2;31(3):329-342.e4. doi: 10.1016/j.str.2022.12.011. Epub 2023 Jan 16. Structure. 2023. PMID: 36649708 Free PMC article.
-
Functional roles of the human ribonuclease A superfamily in RNA metabolism and membrane receptor biology.Mol Aspects Med. 2019 Dec;70:106-116. doi: 10.1016/j.mam.2019.03.003. Epub 2019 Mar 25. Mol Aspects Med. 2019. PMID: 30902663 Free PMC article. Review.
References
-
- Lacy P., Moqbel R. Chapter 7.6: Signaling and degranulation. In: Lee J.J., Rosenberg H.F., editors. Eosinophils in Health and Disease. Elsevier Press; Waltham, MA, USA: 2013. pp. 206–219.
-
- Abu-Ghazaleh R.I., Dunnette S.L., Loegering D.A., Checkel J.L., Kita H., Thomas L.L., Gleich G.J. Eosinophil granule proteins in peripheral blood granulocytes. J. Leukoc. Biol. 1992;52:611–618. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

