AIP1-mediated actin disassembly is required for postnatal germ cell migration and spermatogonial stem cell niche establishment
- PMID: 26181199
- PMCID: PMC4650729
- DOI: 10.1038/cddis.2015.182
AIP1-mediated actin disassembly is required for postnatal germ cell migration and spermatogonial stem cell niche establishment
Abstract
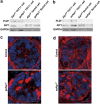
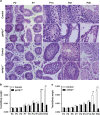
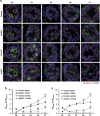
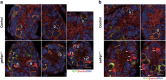
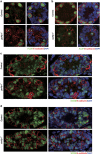
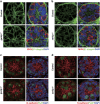
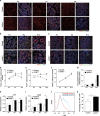
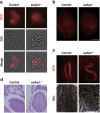
In mammals, spermatogonial stem cells (SSCs) arise from early germ cells called gonocytes, which are derived from primordial germ cells during embryogenesis and remain quiescent until birth. After birth, these germ cells migrate from the center of testicular cord, through Sertoli cells, and toward the basement membrane to form the SSC pool and establish the SSC niche architecture. However, molecular mechanisms underlying germ cell migration and niche establishment are largely unknown. Here, we show that the actin disassembly factor actin interacting protein 1 (AIP1) is required in both germ cells and Sertoli cells to regulate this process. Germ cell-specific or Sertoli cell-specific deletion of Aip1 gene each led to significant defects in germ cell migration after postnatal day 4 or 5, accompanied by elevated levels of actin filaments (F-actin) in the affected cells. Furthermore, our data demonstrated that interaction between germ cells and Sertoli cells, likely through E-cadherin-mediated cell adhesion, is critical for germ cells' migration toward the basement membrane. At last, Aip1 deletion in Sertoli cells decreased SSC self-renewal, increased spermatogonial differentiation, but did not affect the expression and secretion levels of growth factors, suggesting that the disruption of SSC function results from architectural changes in the postnatal niche.
Figures
Similar articles
-
Temporal-Spatial Establishment of Initial Niche for the Primary Spermatogonial Stem Cell Formation Is Determined by an ARID4B Regulatory Network.Stem Cells. 2017 Jun;35(6):1554-1565. doi: 10.1002/stem.2597. Epub 2017 Mar 16. Stem Cells. 2017. PMID: 28207192 Free PMC article.
-
Loss of Gata4 in Sertoli cells impairs the spermatogonial stem cell niche and causes germ cell exhaustion by attenuating chemokine signaling.Oncotarget. 2015 Nov 10;6(35):37012-27. doi: 10.18632/oncotarget.6115. Oncotarget. 2015. PMID: 26473289 Free PMC article.
-
Loss of Etv5 decreases proliferation and RET levels in neonatal mouse testicular germ cells and causes an abnormal first wave of spermatogenesis.Biol Reprod. 2009 Aug;81(2):258-66. doi: 10.1095/biolreprod.108.075200. Epub 2009 Apr 15. Biol Reprod. 2009. PMID: 19369650 Free PMC article.
-
Mammalian gonocyte and spermatogonia differentiation: recent advances and remaining challenges.Reproduction. 2015 Mar;149(3):R139-57. doi: 10.1530/REP-14-0431. Reproduction. 2015. PMID: 25670871 Review.
-
New insights into male gametogenesis: what about the spermatogonial stem cell niche?Folia Histochem Cytobiol. 2007;45(3):141-7. Folia Histochem Cytobiol. 2007. PMID: 17951161 Review.
Cited by
-
Cofilin-mediated actin dynamics promotes actin bundle formation during Drosophila bristle development.Mol Biol Cell. 2016 Aug 15;27(16):2554-64. doi: 10.1091/mbc.E16-02-0084. Epub 2016 Jul 6. Mol Biol Cell. 2016. PMID: 27385345 Free PMC article.
-
The combination of positive anti‑WDR1 antibodies with negative anti‑CFL1 antibodies in serum is a poor prognostic factor for patients with esophageal carcinoma.Med Int (Lond). 2023 Jan 31;3(2):11. doi: 10.3892/mi.2023.71. eCollection 2023 Mar-Apr. Med Int (Lond). 2023. PMID: 36875818 Free PMC article.
-
VWCE Functions as a Tumor Suppressor in Breast Cancer Cells.Front Oncol. 2020 Oct 22;10:586342. doi: 10.3389/fonc.2020.586342. eCollection 2020. Front Oncol. 2020. PMID: 33194737 Free PMC article.
-
Proteomic analysis and miRNA profiling of human testicular endothelial cell-derived exosomes: the potential effects on spermatogenesis.Asian J Androl. 2022 Sep-Oct;24(5):478-486. doi: 10.4103/aja202190. Asian J Androl. 2022. PMID: 34916478 Free PMC article.
-
Cyclin A2 is essential for mouse gonocyte maturation.Cell Cycle. 2020 Jul;19(13):1654-1664. doi: 10.1080/15384101.2020.1762314. Epub 2020 May 18. Cell Cycle. 2020. PMID: 32420805 Free PMC article.
References
-
- 3Huckins C, Clermont Y. Evolution of gonocytes in the rat testis during late embryonic and early postnatal life. Arch Anat Histol Embryol 1968; 1: 341–354. - PubMed
-
- 4Clermont Y, Perey B. Quantitative study of the cell population of the seminiferous tubules in immature rats. Am J Anat 1957; 100: 241–267. - PubMed
-
- 5Culty M. Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res C Embryo Today 2009; 87: 1–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

