ALDH2 Deficiency Promotes Ethanol-Induced Gut Barrier Dysfunction and Fatty Liver in Mice
- PMID: 26173414
- PMCID: PMC4515212
- DOI: 10.1111/acer.12777
ALDH2 Deficiency Promotes Ethanol-Induced Gut Barrier Dysfunction and Fatty Liver in Mice
Abstract
Background: Acetaldehyde, the toxic ethanol (EtOH) metabolite, disrupts intestinal epithelial barrier function. Aldehyde dehydrogenase (ALDH) detoxifies acetaldehyde into acetate. Subpopulations of Asians and Native Americans show polymorphism with loss-of-function mutations in ALDH2. We evaluated the effect of ALDH2 deficiency on EtOH-induced disruption of intestinal epithelial tight junctions and adherens junctions, gut barrier dysfunction, and liver injury.
Methods: Wild-type and ALDH2-deficient mice were fed EtOH (1 to 6%) in Lieber-DeCarli diet for 4 weeks. Gut permeability in vivo was measured by plasma-to-luminal flux of FITC-inulin, tight junction and adherens junction integrity was analyzed by confocal microscopy, and liver injury was assessed by the analysis of plasma transaminase activity, histopathology, and liver triglyceride.
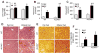
Results: EtOH feeding elevated colonic mucosal acetaldehyde, which was significantly greater in ALDH2-deficient mice. ALDH2(-/-) mice showed a drastic reduction in the EtOH diet intake. Therefore, this study was continued only in wild-type and ALDH2(+/-) mice. EtOH feeding elevated mucosal inulin permeability in distal colon, but not in proximal colon, ileum, or jejunum of wild-type mice. In ALDH2(+/-) mice, EtOH-induced inulin permeability in distal colon was not only higher than that in wild-type mice, but inulin permeability was also elevated in the proximal colon, ileum, and jejunum. Greater inulin permeability in distal colon of ALDH2(+/-) mice was associated with a more severe redistribution of tight junction and adherens junction proteins from the intercellular junctions. In ALDH2(+/-) mice, but not in wild-type mice, EtOH feeding caused a loss of junctional distribution of tight junction and adherens junction proteins in the ileum. Histopathology, plasma transaminases, and liver triglyceride analyses showed that EtOH-induced liver damage was significantly greater in ALDH2(+/-) mice compared to wild-type mice.
Conclusions: These data demonstrate that ALDH2 deficiency enhances EtOH-induced disruption of intestinal epithelial tight junctions, barrier dysfunction, and liver damage.
Keywords: Acetaldehyde; Adherens Junction; Alcohol; Gut Permeability; Tight Junction.
Copyright © 2015 by the Research Society on Alcoholism.
Figures
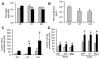
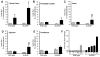




Similar articles
-
Occludin deficiency promotes ethanol-induced disruption of colonic epithelial junctions, gut barrier dysfunction and liver damage in mice.Biochim Biophys Acta. 2016 Apr;1860(4):765-74. doi: 10.1016/j.bbagen.2015.12.013. Epub 2015 Dec 23. Biochim Biophys Acta. 2016. PMID: 26721332 Free PMC article.
-
Glutamine supplementation attenuates ethanol-induced disruption of apical junctional complexes in colonic epithelium and ameliorates gut barrier dysfunction and fatty liver in mice.J Nutr Biochem. 2016 Jan;27:16-26. doi: 10.1016/j.jnutbio.2015.08.012. Epub 2015 Aug 20. J Nutr Biochem. 2016. PMID: 26365579 Free PMC article.
-
EGF receptor plays a role in the mechanism of glutamine-mediated prevention of alcohol-induced gut barrier dysfunction and liver injury.J Nutr Biochem. 2019 Feb;64:128-143. doi: 10.1016/j.jnutbio.2018.10.016. Epub 2018 Nov 6. J Nutr Biochem. 2019. PMID: 30502657 Free PMC article.
-
Characteristics of aldehyde dehydrogenase 2 (Aldh2) knockout mice.Toxicol Mech Methods. 2009 Nov;19(9):535-40. doi: 10.3109/15376510903401708. Toxicol Mech Methods. 2009. PMID: 19874182 Review.
-
Enteric Pathogens and Their Toxin-Induced Disruption of the Intestinal Barrier through Alteration of Tight Junctions in Chickens.Toxins (Basel). 2017 Feb 10;9(2):60. doi: 10.3390/toxins9020060. Toxins (Basel). 2017. PMID: 28208612 Free PMC article. Review.
Cited by
-
Deletion of TLR-4 attenuates fetal alcohol exposure-induced gene expression and social interaction deficits.Alcohol. 2018 Dec;73:73-78. doi: 10.1016/j.alcohol.2018.04.004. Epub 2018 Apr 18. Alcohol. 2018. PMID: 30312858 Free PMC article.
-
Aldehyde Dehydrogenase, Liver Disease and Cancer.Int J Biol Sci. 2020 Jan 22;16(6):921-934. doi: 10.7150/ijbs.42300. eCollection 2020. Int J Biol Sci. 2020. PMID: 32140062 Free PMC article. Review.
-
Skin Immunization Obviates Alcohol-Related Immune Dysfunction.Biomolecules. 2015 Nov 6;5(4):3009-28. doi: 10.3390/biom5043009. Biomolecules. 2015. PMID: 26561838 Free PMC article.
-
The interplay of pineal hormones and socioeconomic status leading to colorectal cancer disparity.Transl Oncol. 2022 Feb;16:101330. doi: 10.1016/j.tranon.2021.101330. Epub 2022 Jan 3. Transl Oncol. 2022. PMID: 34990909 Free PMC article. Review.
-
Different Dietary Proportions of Fish Oil Regulate Inflammatory Factors but Do Not Change Intestinal Tight Junction ZO-1 Expression in Ethanol-Fed Rats.Mediators Inflamm. 2017;2017:5801768. doi: 10.1155/2017/5801768. Epub 2017 Dec 13. Mediators Inflamm. 2017. PMID: 29386752 Free PMC article.
References
-
- ANDERSON C, ANDERSSON T, MOLANDER M. Ethanol absorption across human skin measured by in vivo microdialysis technique. Acta Derm Venereol. 1991;71:389–93. - PubMed
-
- ATKINSON KJ, RAO RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1280–8. - PubMed
-
- BASUROY S, SHETH P, MANSBACH CM, RAO RK. Acetaldehyde disrupts tight junctions and adherens junctions in human colonic mucosa: protection by EGF and L-glutamine. American journal of physiology Gastrointestinal and liver physiology. 2005;289:G367–75. - PubMed
-
- BODE JC, BODE C, HEIDELBACH R, DURR HK, MARTINI GA. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology. 1984;31:30–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

