Modeling HIV-1 Latency in Primary T Cells Using a Replication-Competent Virus
- PMID: 26171776
- PMCID: PMC4761834
- DOI: 10.1089/aid.2015.0106
Modeling HIV-1 Latency in Primary T Cells Using a Replication-Competent Virus
Abstract
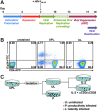
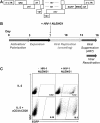
HIV-1 latently infected cells in vivo can be found in extremely low frequencies. Therefore, in vitro cell culture models have been used extensively for the study of HIV-1 latency. Often, these in vitro systems utilize defective viruses. Defective viruses allow for synchronized infections and circumvent the use of antiretrovirals. In addition, replication-defective viruses cause minimal cytopathicity because they fail to spread and usually do not encode env or accessory genes. On the other hand, replication-competent viruses encode all or most viral genes and better recapitulate the nuances of the viral replication cycle. The study of latency with replication-competent viruses requires the use of antiretroviral drugs in culture, and this mirrors the use of antiretroviral treatment (ART) in vivo. We describe a model that utilizes cultured central memory CD4(+) T cells and replication-competent HIV-1. This method generates latently infected cells that can be reactivated using latency reversing agents in the presence of antiretroviral drugs. We also describe a method for the removal of productively infected cells prior to viral reactivation, which takes advantage of the downregulation of CD4 by HIV-1, and the use of a GFP-encoding virus for increased throughput.
Figures

Similar articles
-
Modeling HIV-1 Latency Using Primary CD4+ T Cells from Virally Suppressed HIV-1-Infected Individuals on Antiretroviral Therapy.J Virol. 2019 May 15;93(11):e02248-18. doi: 10.1128/JVI.02248-18. Print 2019 Jun 1. J Virol. 2019. PMID: 30918072 Free PMC article.
-
In Vitro Reactivation of Replication-Competent and Infectious HIV-1 by Histone Deacetylase Inhibitors.J Virol. 2015 Dec 9;90(4):1858-71. doi: 10.1128/JVI.02359-15. Print 2016 Feb 15. J Virol. 2015. PMID: 26656693 Free PMC article.
-
The Pathway To Establishing HIV Latency Is Critical to How Latency Is Maintained and Reversed.J Virol. 2018 Jun 13;92(13):e02225-17. doi: 10.1128/JVI.02225-17. Print 2018 Jul 1. J Virol. 2018. PMID: 29643247 Free PMC article.
-
Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy.Annu Rev Immunol. 2000;18:665-708. doi: 10.1146/annurev.immunol.18.1.665. Annu Rev Immunol. 2000. PMID: 10837072 Review.
-
The challenge of viral reservoirs in HIV-1 infection.Annu Rev Med. 2002;53:557-93. doi: 10.1146/annurev.med.53.082901.104024. Annu Rev Med. 2002. PMID: 11818490 Review.
Cited by
-
A Matter of Life or Death: Productively Infected and Bystander CD4 T Cells in Early HIV Infection.Front Immunol. 2021 Feb 12;11:626431. doi: 10.3389/fimmu.2020.626431. eCollection 2020. Front Immunol. 2021. PMID: 33643305 Free PMC article.
-
Discovery of candidate HIV-1 latency biomarkers using an OMICs approach.Virology. 2021 Jun;558:86-95. doi: 10.1016/j.virol.2021.03.003. Epub 2021 Mar 11. Virology. 2021. PMID: 33735754 Free PMC article.
-
Virally Suppressed People Living with HIV Who Use Opioids Have Diminished Latency Reversal.Viruses. 2023 Feb 1;15(2):415. doi: 10.3390/v15020415. Viruses. 2023. PMID: 36851631 Free PMC article.
-
Kinetics of Early Innate Immune Activation during HIV-1 Infection of Humanized Mice.J Virol. 2019 May 15;93(11):e02123-18. doi: 10.1128/JVI.02123-18. Print 2019 Jun 1. J Virol. 2019. PMID: 30867315 Free PMC article.
-
Single-molecule RNA-FISH analysis reveals stochasticity in reactivation of latent HIV-1 regulated by Nuclear Orphan Receptors NR4A and cMYC.Res Sq [Preprint]. 2024 Apr 19:rs.3.rs-4166090. doi: 10.21203/rs.3.rs-4166090/v1. Res Sq. 2024. PMID: 38699331 Free PMC article. Preprint.
References
-
- Finzi D, Hermankova M, Pierson T, et al. : Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997;278(5341):1295–1300 - PubMed
-
- Chun TW, Carruth L, Finzi D, et al. : Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 1997;387(6629):183–188 - PubMed
-
- Wong JK, Hezareh M, Gunthard HF, et al. : Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 1997;278(5341):1291–1295 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

