Orientia tsutsugamushi Strain Ikeda Ankyrin Repeat-Containing Proteins Recruit SCF1 Ubiquitin Ligase Machinery via Poxvirus-Like F-Box Motifs
- PMID: 26170417
- PMCID: PMC4560276
- DOI: 10.1128/JB.00276-15
Orientia tsutsugamushi Strain Ikeda Ankyrin Repeat-Containing Proteins Recruit SCF1 Ubiquitin Ligase Machinery via Poxvirus-Like F-Box Motifs
Abstract
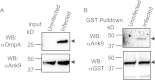
A rising theme among intracellular microbes is the delivery of ankyrin repeat-containing effectors (Anks) that interact with target proteins to co-opt host cell functions. Orientia tsutsugamushi, an obligate intracellular bacterium and the etiologic agent of scrub typhus, encodes one of the largest Ank repertoires of any sequenced microorganism. They have been previously identified as type 1 secretion system substrates. Here, in silico and manual sequence analyses revealed that a large proportion of O. tsutsugamushi strain Ikeda Anks bear a eukaryotic/poxvirus-like F-box motif, which is known to recruit host cell SCF1 ubiquitin ligase machinery. We assessed the Anks for the ability to serve as F-box proteins. Coimmunoprecipitation assays demonstrated that F-box-containing Anks interact with overexpressed and/or endogenous SCF1 components. When coexpressed with FLAG-Ank4_01 or FLAG-Ank9, a glutathione S-transferase (GST)-tagged version of the SCF1 component SKP1 localized to subcellular sites of FLAG-Ank accumulation. The abilities of recombinant Anks to interact and colocalize with SKP1 were F-box dependent. GST-SKP1 precipitated O. tsutsugamushi-derived Ank9 from infected host cells, verifying both that the pathogen expresses Ank9 during infection and the protein's capability to bind SKP1. Aligning O. tsutsugamushi, poxviral, and eukaryotic F-box sequences delineated three F-box residues that are highly conserved and likely to be functionally important. Substitution of these residues ablated the ability of GFP-Ank9 to interact with GST-SKP1. These results demonstrate that O. tsutsugamushi strain Ikeda Anks can co-opt host cell polyubiquitination machinery, provide the first evidence that an O. tsutsugamushi Ank does so during infection, and advance overall understanding of microbial F-box proteins.
Importance: Ankyrin repeat-containing proteins (Anks) are important virulence factors of intracellular bacteria that mediate protein-protein interactions with host cell targets. Orientia tsutsugamushi, which causes a debilitating infection called scrub typhus in one of the most densely populated regions of the world, encodes one of the largest Ank armamentariums of any sequenced bacterium. This study demonstrates that O. tsutsugamushi strain Ikeda Anks also bear F-box motifs that interact with host cell polyubiquitination machinery. By proving that an Orientia-derived Ank interacts with SKP1 in infected cells, this evidences the first bona fide Orientia effector and the first example of an endogenous F-box-containing Ank-mammalian-host ligand interaction for any intracellular bacterium. Also, importantly, this work identifies key residues that are essential for microbial F-box function.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures






Similar articles
-
Functional Characterization of Non-Ankyrin Repeat Domains of Orientia tsutsugamushi Ank Effectors Reveals Their Importance for Molecular Pathogenesis.Infect Immun. 2022 May 19;90(5):e0062821. doi: 10.1128/iai.00628-21. Epub 2022 Apr 18. Infect Immun. 2022. PMID: 35435726 Free PMC article.
-
Orientia tsutsugamushi ankyrin repeat-containing protein family members are Type 1 secretion system substrates that traffic to the host cell endoplasmic reticulum.Front Cell Infect Microbiol. 2015 Feb 3;4:186. doi: 10.3389/fcimb.2014.00186. eCollection 2014. Front Cell Infect Microbiol. 2015. PMID: 25692099 Free PMC article.
-
Multiple Orientia tsutsugamushi ankyrin repeat proteins interact with SCF1 ubiquitin ligase complex and eukaryotic elongation factor 1 α.PLoS One. 2014 Aug 28;9(8):e105652. doi: 10.1371/journal.pone.0105652. eCollection 2014. PLoS One. 2014. PMID: 25166298 Free PMC article.
-
Poxviral ankyrin proteins.Viruses. 2015 Feb 16;7(2):709-38. doi: 10.3390/v7020709. Viruses. 2015. PMID: 25690795 Free PMC article. Review.
-
Subversion of host cell signaling by Orientia tsutsugamushi.Microbes Infect. 2011 Jul;13(7):638-48. doi: 10.1016/j.micinf.2011.03.003. Epub 2011 Mar 31. Microbes Infect. 2011. PMID: 21458586 Review.
Cited by
-
Comparative transcriptomic analysis of Rickettsia conorii during in vitro infection of human and tick host cells.BMC Genomics. 2020 Sep 25;21(1):665. doi: 10.1186/s12864-020-07077-w. BMC Genomics. 2020. PMID: 32977742 Free PMC article.
-
Orientia tsutsugamushi: analysis of the mobilome of a highly fragmented and repetitive genome reveals ongoing lateral gene transfer in an obligate intracellular bacterium.bioRxiv [Preprint]. 2023 May 11:2023.05.11.540415. doi: 10.1101/2023.05.11.540415. bioRxiv. 2023. Update in: mSphere. 2023 Dec 20;8(6):e0026823. doi: 10.1128/msphere.00268-23. PMID: 37215039 Free PMC article. Updated. Preprint.
-
Subversion of the Endocytic and Secretory Pathways by Bacterial Effector Proteins.Front Cell Dev Biol. 2018 Jan 24;6:1. doi: 10.3389/fcell.2018.00001. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 29417046 Free PMC article. Review.
-
Orientia tsutsugamushi Modulates Endoplasmic Reticulum-Associated Degradation To Benefit Its Growth.Infect Immun. 2017 Dec 19;86(1):e00596-17. doi: 10.1128/IAI.00596-17. Print 2018 Jan. Infect Immun. 2017. PMID: 29109174 Free PMC article.
-
Orientia tsutsugamushi infection reduces host gluconeogenic but not glycolytic substrates.Infect Immun. 2024 Nov 12;92(11):e0028424. doi: 10.1128/iai.00284-24. Epub 2024 Sep 26. Infect Immun. 2024. PMID: 39324805
References
-
- Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, Jones M, Jenjaroen K, Vongsouvath M, Ferguson DP, Blacksell SD, Newton PN, Day NP, Turner GD. 2012. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis 6:e1466. doi:10.1371/journal.pntd.0001466. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

