Arf6 controls beta-amyloid production by regulating macropinocytosis of the Amyloid Precursor Protein to lysosomes
- PMID: 26170135
- PMCID: PMC4501290
- DOI: 10.1186/s13041-015-0129-7
Arf6 controls beta-amyloid production by regulating macropinocytosis of the Amyloid Precursor Protein to lysosomes
Abstract
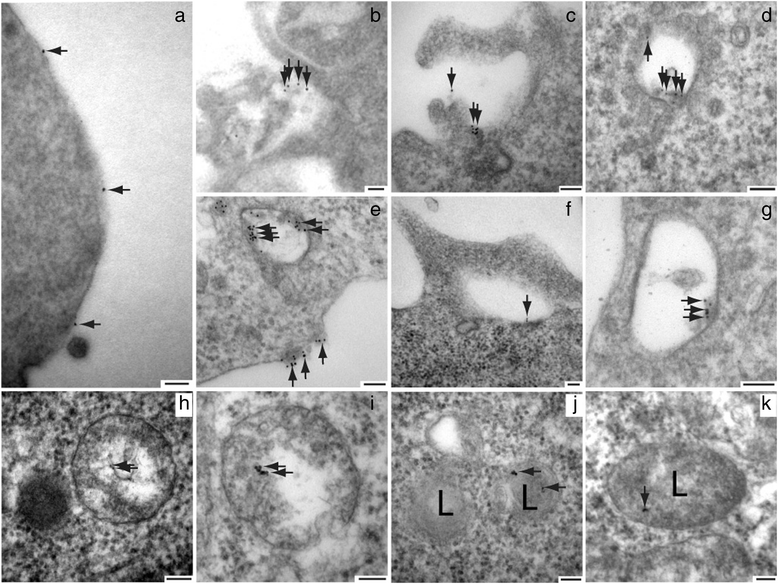
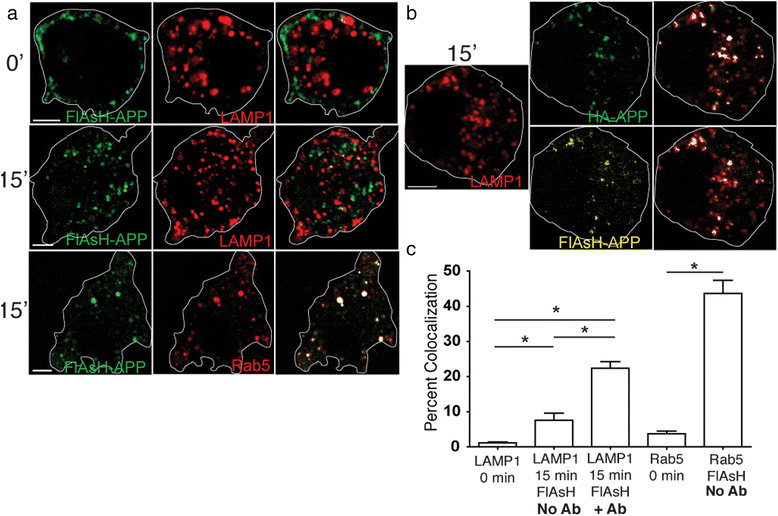
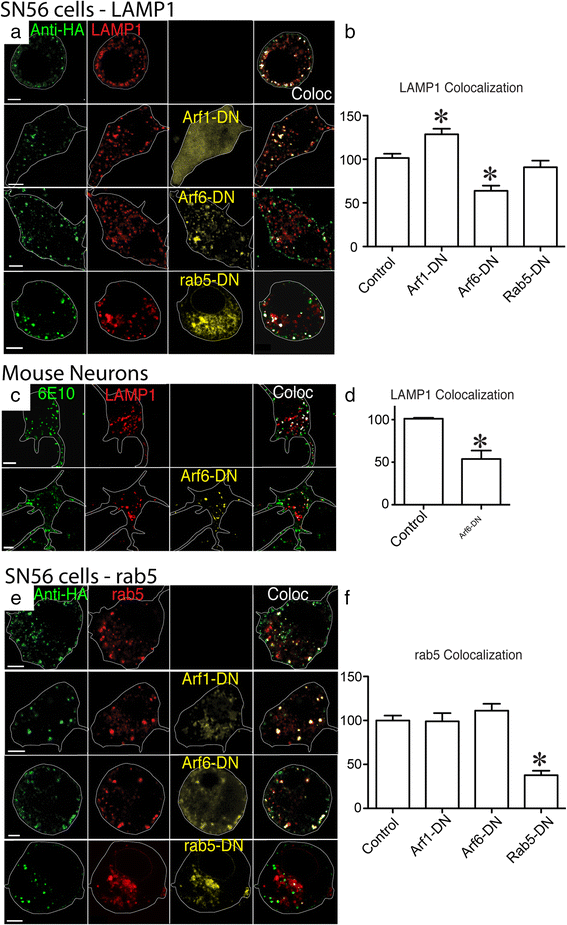
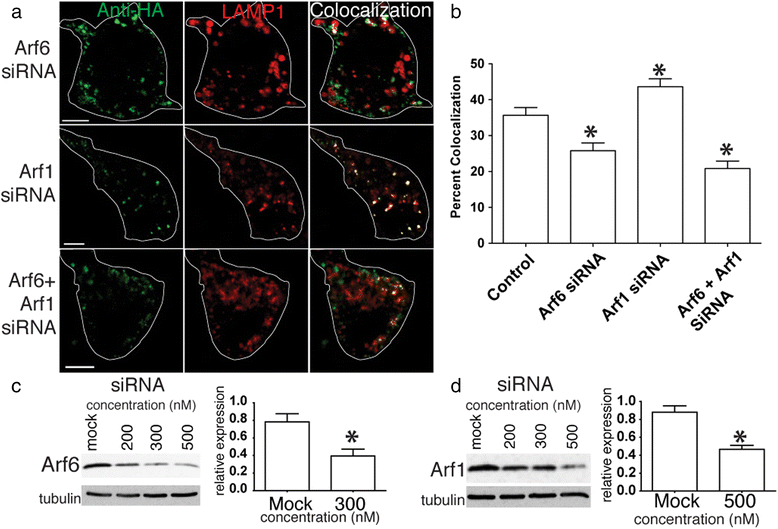
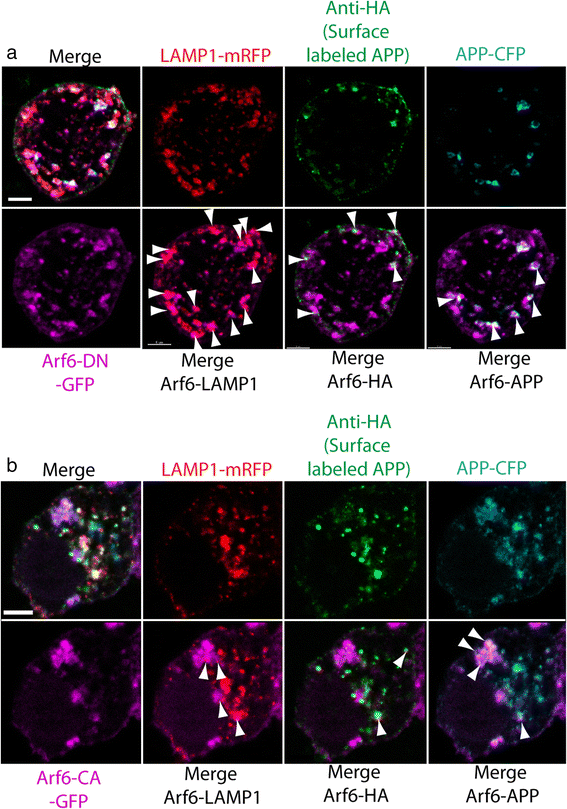
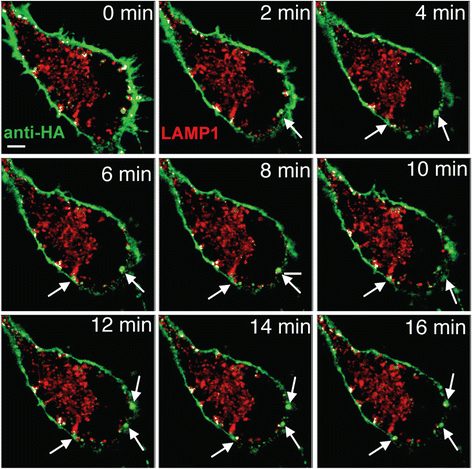
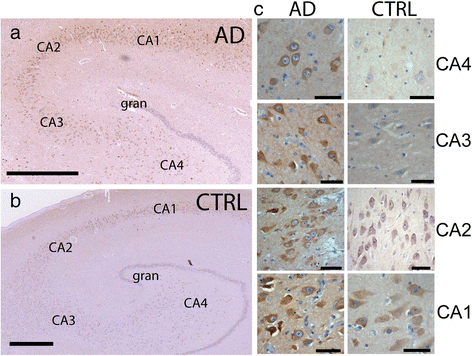
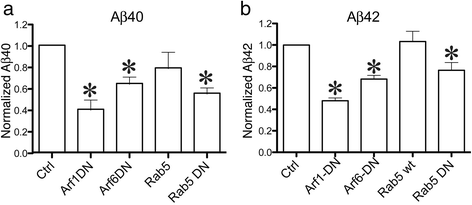
Alzheimer's disease (AD) is characterized by the deposition of Beta-Amyloid (Aβ) peptides in the brain. Aβ peptides are generated by cleavage of the Amyloid Precursor Protein (APP) by the β - and γ - secretase enzymes. Although this process is tightly linked to the internalization of cell surface APP, the compartments responsible are not well defined. We have found that APP can be rapidly internalized from the cell surface to lysosomes, bypassing early and late endosomes. Here we show by confocal microscopy and electron microscopy that this pathway is mediated by macropinocytosis. APP internalization is enhanced by antibody binding/crosslinking of APP suggesting that APP may function as a receptor. Furthermore, a dominant negative mutant of Arf6 blocks direct transport of APP to lysosomes, but does not affect classical endocytosis to endosomes. Arf6 expression increases through the hippocampus with the development of Alzheimer's disease, being expressed mostly in the CA1 and CA2 regions in normal individuals but spreading through the CA3 and CA4 regions in individuals with pathologically diagnosed AD. Disruption of lysosomal transport of APP reduces both Aβ40 and Aβ42 production by more than 30 %. Our findings suggest that the lysosome is an important site for Aβ production and that altering APP trafficking represents a viable strategy to reduce Aβ production.
Figures
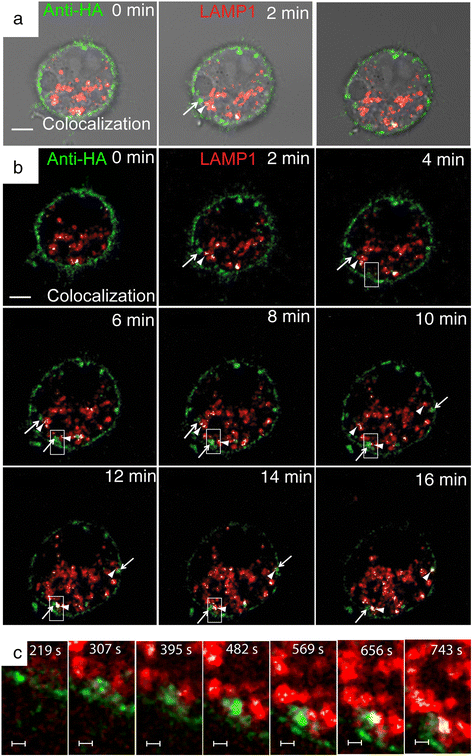
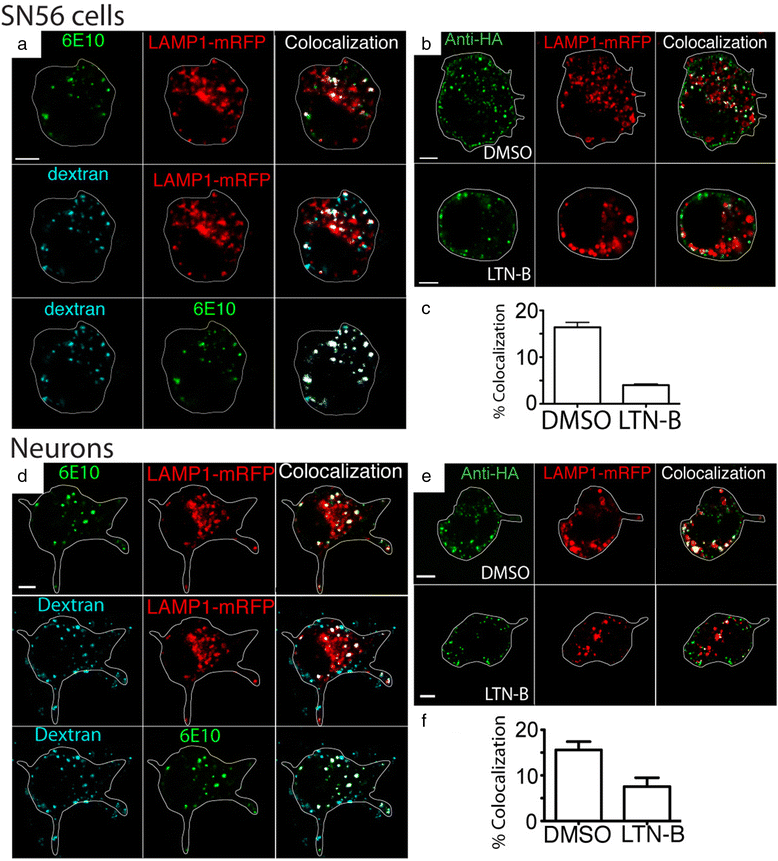
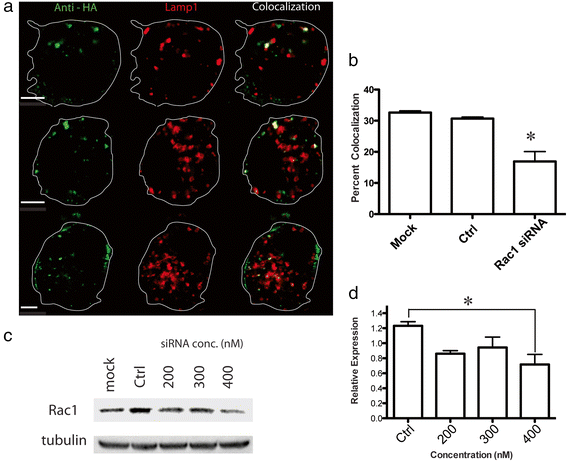
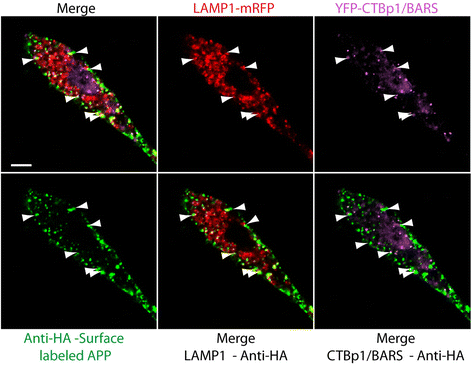
Similar articles
-
The Amyloid Precursor Protein is rapidly transported from the Golgi apparatus to the lysosome and where it is processed into beta-amyloid.Mol Brain. 2014 Aug 1;7:54. doi: 10.1186/s13041-014-0054-1. Mol Brain. 2014. PMID: 25085554 Free PMC article.
-
ADP ribosylation factor 6 (ARF6) controls amyloid precursor protein (APP) processing by mediating the endosomal sorting of BACE1.Proc Natl Acad Sci U S A. 2011 Aug 23;108(34):E559-68. doi: 10.1073/pnas.1100745108. Epub 2011 Aug 8. Proc Natl Acad Sci U S A. 2011. PMID: 21825135 Free PMC article.
-
Amyloid precursor protein traffics from the Golgi directly to early endosomes in an Arl5b- and AP4-dependent pathway.Traffic. 2017 Mar;18(3):159-175. doi: 10.1111/tra.12465. Epub 2017 Jan 30. Traffic. 2017. PMID: 28000370
-
Proton myo-inositol cotransporter is a novel γ-secretase associated protein that regulates Aβ production without affecting Notch cleavage.FEBS J. 2015 Sep;282(17):3438-51. doi: 10.1111/febs.13353. Epub 2015 Jul 14. FEBS J. 2015. PMID: 26094765 Review.
-
Amyloid precursor protein and endosomal-lysosomal dysfunction in Alzheimer's disease: inseparable partners in a multifactorial disease.FASEB J. 2017 Jul;31(7):2729-2743. doi: 10.1096/fj.201700359. FASEB J. 2017. PMID: 28663518 Free PMC article. Review.
Cited by
-
Daily Brief Heat Therapy Reduces Seizures in A350V IQSEC2 Mice and Is Associated with Correction of AMPA Receptor-Mediated Synaptic Dysfunction.Int J Mol Sci. 2023 Feb 15;24(4):3924. doi: 10.3390/ijms24043924. Int J Mol Sci. 2023. PMID: 36835332 Free PMC article.
-
Direct imaging of APP proteolysis in living cells.PeerJ. 2017 Apr 12;5:e3086. doi: 10.7717/peerj.3086. eCollection 2017. PeerJ. 2017. PMID: 28413720 Free PMC article.
-
Amyloid Beta Is Internalized via Macropinocytosis, an HSPG- and Lipid Raft-Dependent and Rac1-Mediated Process.Front Mol Neurosci. 2022 Feb 11;15:804702. doi: 10.3389/fnmol.2022.804702. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36187354 Free PMC article.
-
Physiological and Pathological Roles of the Cytohesin Family in Neurons.Int J Mol Sci. 2022 May 3;23(9):5087. doi: 10.3390/ijms23095087. Int J Mol Sci. 2022. PMID: 35563476 Free PMC article. Review.
-
APP Protein Family Signaling at the Synapse: Insights from Intracellular APP-Binding Proteins.Front Mol Neurosci. 2017 Mar 30;10:87. doi: 10.3389/fnmol.2017.00087. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28424586 Free PMC article. Review.
References
-
- Pasternak SH, Callahan JW, Mahuran DJ. The role of the endosomal/lysosomal system in amyloid-beta production and the pathophysiology of Alzheimer’s disease: Reexamining the spatial paradox from a lysosomal perspective. J Alzheimers Dis. 2004;6(1):53–65. - PubMed
-
- Grbovic OM, Mathews PM, Jiang Y, Schmidt SD, Dinakar R, Summers-Terio NB, et al. Rab5-stimulated up-regulation of the endocytic pathway increases intracellular beta-cleaved amyloid precursor protein carboxyl-terminal fragment levels and Abeta production. J Biol Chem. 2003;278(33):31261–8. doi: 10.1074/jbc.M304122200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

